Abstract
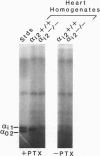
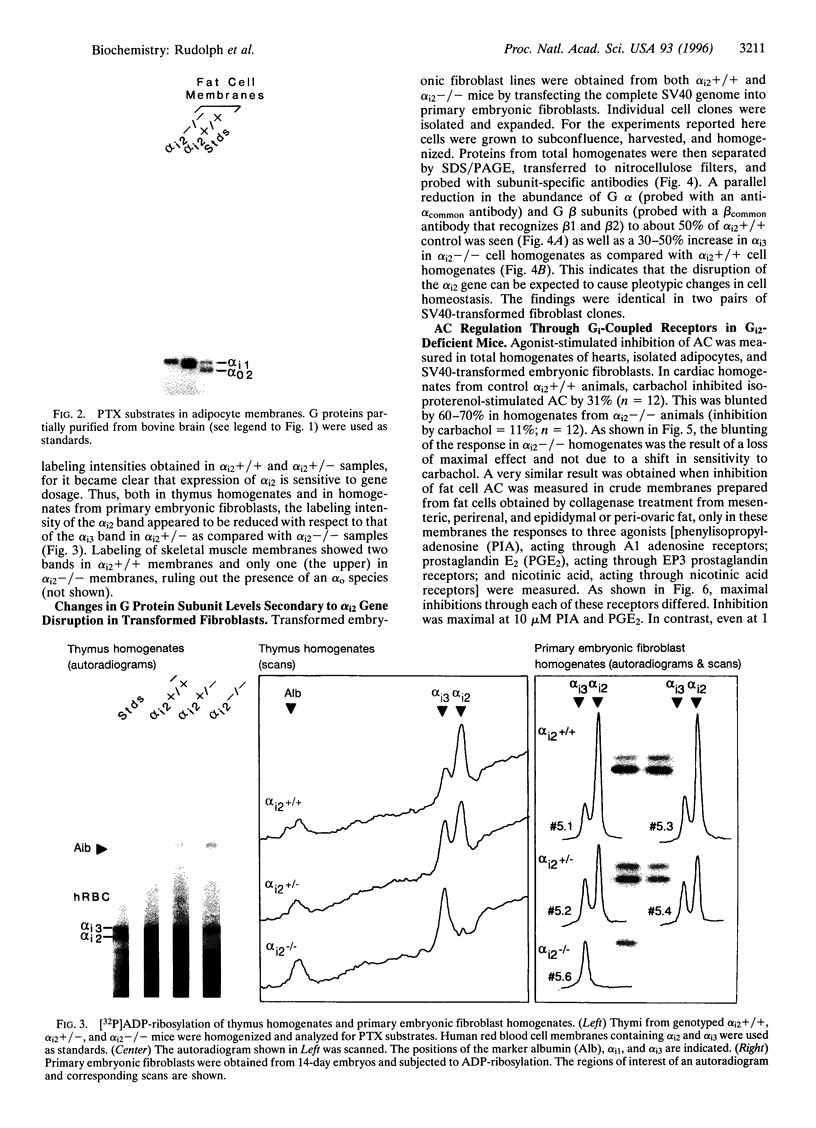
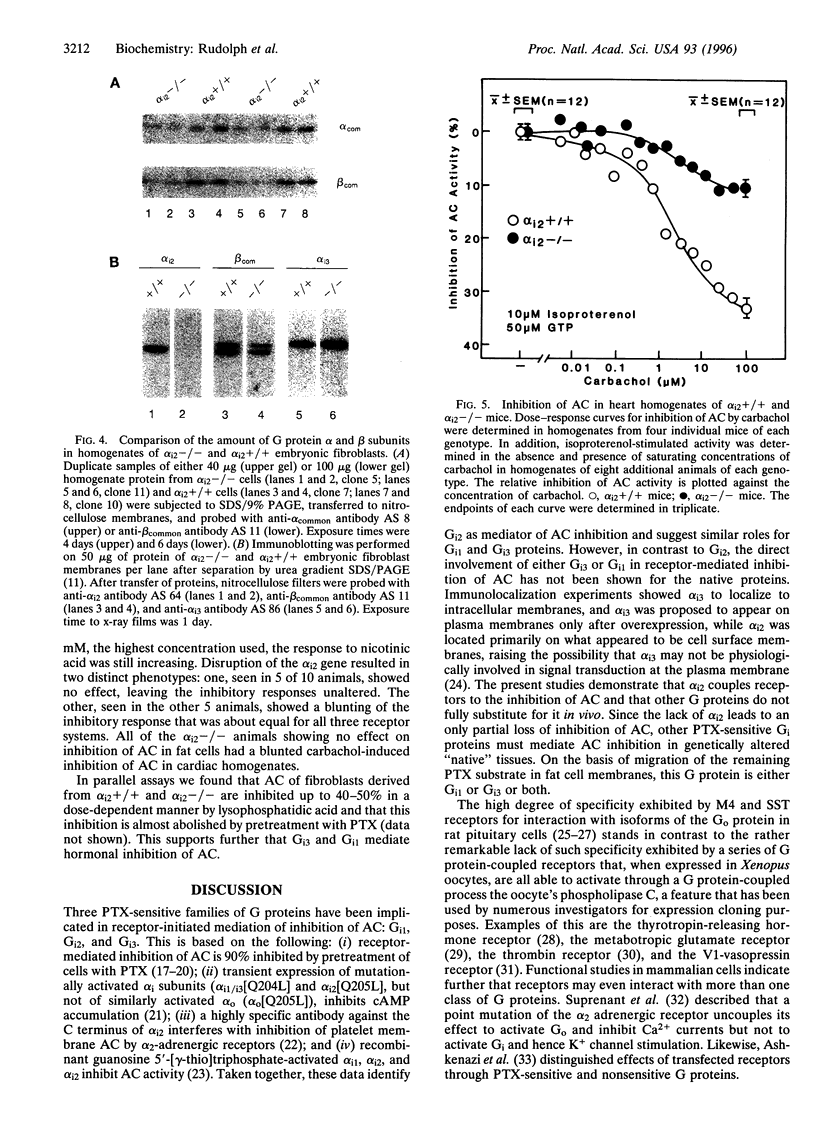
The inhibition of alpha i2-/- mouse cardiac isoproterenol-stimulated adenylyl cyclase (AC; EC 4.6.1.1) activity by carbachol and that of alpha i2-/- adipocyte AC by phenylisopropyladenosine (PIA), prostaglandin E2, and nicotinic acid were partially, but not completely, inhibited. While the inhibition of cardiac AC was affected in all alpha i2-/- animals tested, only 50% of the alpha i2-/- animals showed an impaired inhibition of adipocyte AC, indicative of a partial penetrance of this phenotype. In agreement with previous results, the data show that Gi2 mediates hormonal inhibition of AC and that Gi3 and/or Gi1 is capable of doing the same but with a lower efficacy. Disruption of the alpha i2 gene affected about equally the actions of all the receptors studied, indicating that none of them exhibits a striking specificity for one type of Gi over another and that receptors are likely to he selective rather than specific in their interaction with functionally homologous G proteins (e.g., Gi1, Gi2, Gi3). Western analysis of G protein subunit levels in simian virus 40-transformed primary embryonic fibroblasts from alpha i2+/+ and alpha i2-/- animals showed that alpha i2 accounts for about 50% of the immunopositive G protein alpha subunits and that loss of the alpha i2 is accompanied by a parallel reduction in G beta 35 and G beta 36 subunits and by a 30-50% increase in alpha i3. This suggests that G beta-gamma levels may be regulated passively through differential rates of turnover in their free vs. trimeric states. The existence of compensatory increase(s) in alpha i subunit expression raises the possibility that the lack of effect of a missing alpha i2 on AC inhibition in adipocytes of some alpha i2-/- animals may be the reflection of a more pronounced compensatory expression of alpha i3 and/or alpha i1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Codina J., Grenet D., Chang K. J., Birnbaumer L. Urea gradient/SDS-PAGE; a useful tool in the investigation of signal transducing G proteins. J Recept Res. 1991;11(1-4):587–601. doi: 10.3109/10799899109066429. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Hermouet S., Murakami T., Spiegel A. M. Stable changes in expression or activation of G protein alpha i or alpha q subunits affect the expression of both beta 1 and beta 2 subunits. FEBS Lett. 1993 Jul 26;327(2):183–188. doi: 10.1016/0014-5793(93)80166-r. [DOI] [PubMed] [Google Scholar]
- Hermouet S., de Mazancourt P., Spiegel A. M., Farquhar M. G., Wilson B. S. High level expression of transfected G protein alpha i3 subunit is required for plasma membrane targeting and adenylyl cyclase inhibition in NIH 3T3 fibroblasts. FEBS Lett. 1992 Nov 9;312(2-3):223–228. doi: 10.1016/0014-5793(92)80940-i. [DOI] [PubMed] [Google Scholar]
- Hinsch K. D., Rosenthal W., Spicher K., Binder T., Gausepohl H., Frank R., Schultz G., Joost H. G. Adipocyte plasma membranes contain two Gi subtypes but are devoid of Go. FEBS Lett. 1988 Sep 26;238(1):191–196. doi: 10.1016/0014-5793(88)80254-0. [DOI] [PubMed] [Google Scholar]
- Hinsch K. D., Tychowiecka I., Gausepohl H., Frank R., Rosenthal W., Schultz G. Tissue distribution of beta 1- and beta 2-subunits of regulatory guanine nucleotide-binding proteins. Biochim Biophys Acta. 1989 Sep 4;1013(1):60–67. doi: 10.1016/0167-4889(89)90128-6. [DOI] [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Hsu W. H., Rudolph U., Sanford J., Bertrand P., Olate J., Nelson C., Moss L. G., Boyd A. E., Codina J., Birnbaumer L. Molecular cloning of a novel splice variant of the alpha subunit of the mammalian Go protein. J Biol Chem. 1990 Jul 5;265(19):11220–11226. [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992 Jul 30;358(6385):424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Kurose H., Ui M. Functional uncoupling of muscarinic receptors from adenylate cyclase in rat cardiac membranes by the active component of islet-activating protein, pertussis toxin. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):305–318. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy F. O., Gudermann T., Perez-Reyes E., Birnbaumer M., Kaumann A. J., Birnbaumer L. Molecular cloning of a human serotonin receptor (S12) with a pharmacological profile resembling that of the 5-HT1D subtype. J Biol Chem. 1992 Apr 15;267(11):7553–7562. [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. [3H]GDP release from rat and hamster adipocyte membranes independently linked to receptors involved in activation or inhibition of adenylate cyclase. Differential susceptibility to two bacterial toxins. J Biol Chem. 1984 Jan 25;259(2):761–769. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rosenthal W., Koesling D., Rudolph U., Kleuss C., Pallast M., Yajima M., Schultz G. Identification and characterization of the 35-kDa beta subunit of guanine-nucleotide-binding proteins by an antiserum raised against transducin. Eur J Biochem. 1986 Jul 15;158(2):255–263. doi: 10.1111/j.1432-1033.1986.tb09745.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Brabet P., Hasty P., Bradley A., Birnbaumer L. Disruption of the G(i2) alpha locus in embryonic stem cells and mice: a modified hit and run strategy with detection by a PCR dependent on gap repair. Transgenic Res. 1993 Nov;2(6):345–355. doi: 10.1007/BF01976176. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Finegold M. J., Rich S. S., Harriman G. R., Srinivasan Y., Brabet P., Boulay G., Bradley A., Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995 Jun;10(2):143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Scherer N. M., Toro M. J., Entman M. L., Birnbaumer L. G-protein distribution in canine cardiac sarcoplasmic reticulum and sarcolemma: comparison to rabbit skeletal muscle membranes and to brain and erythrocyte G-proteins. Arch Biochem Biophys. 1987 Dec;259(2):431–440. doi: 10.1016/0003-9861(87)90509-1. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicher K., Kalkbrenner F., Zobel A., Harhammer R., Nürnberg B., Söling A., Schultz G. G12 and G13 alpha-subunits are immunochemically detectable in most membranes of various mammalian cells and tissues. Biochem Biophys Res Commun. 1994 Feb 15;198(3):906–914. doi: 10.1006/bbrc.1994.1129. [DOI] [PubMed] [Google Scholar]
- Straub R. E., Frech G. C., Joho R. H., Gershengorn M. C. Expression cloning of a cDNA encoding the mouse pituitary thyrotropin-releasing hormone receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9514–9518. doi: 10.1073/pnas.87.24.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Horstman D. A., Akbarali H., Limbird L. E. A point mutation of the alpha 2-adrenoceptor that blocks coupling to potassium but not calcium currents. Science. 1992 Aug 14;257(5072):977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- Taussig R., Iñiguez-Lluhi J. A., Gilman A. G. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993 Jul 9;261(5118):218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- Toutant M., Gabrion J., Vandaele S., Peraldi-Roux S., Barhanin J., Bockaert J., Rouot B. Cellular distribution and biochemical characterization of G proteins in skeletal muscle: comparative location with voltage-dependent calcium channels. EMBO J. 1990 Feb;9(2):363–369. doi: 10.1002/j.1460-2075.1990.tb08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Watkins D. C., Moxham C. M., Morris A. J., Malbon C. C. Suppression of Gi alpha 2 enhances phospholipase C signalling. Biochem J. 1994 May 1;299(Pt 3):593–596. doi: 10.1042/bj2990593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. H., Conklin B. R., Bourne H. R. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992 Jan 17;255(5042):339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]