Figure 2. Binding of glutamate to EAAT3 with a protonated vs deprotonated E374 side chain.
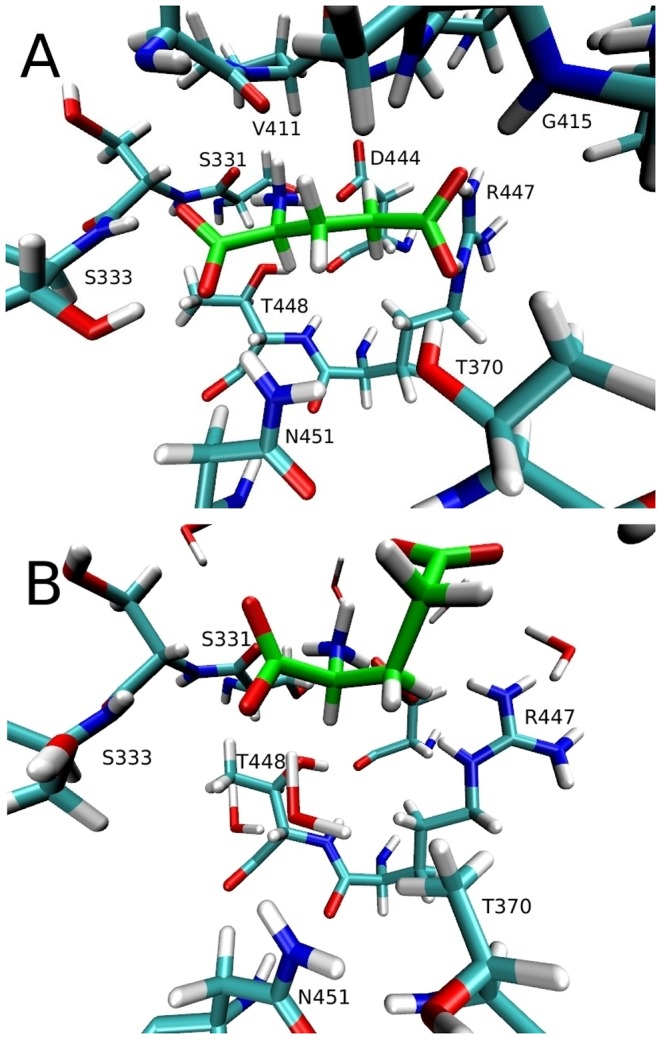
(A) Glutamate substrate (in green) bound to EAAT3 in the closed outward state with a protonated E374 side chain. (B) The same but with a deprotonated E374 side chain. Glutamate is stable in A for 20 ns, but becomes unstable in B, losing most of the contacts after 10 ns of simulations.
