Figure 5. Effect of the D455N mutation on sodium binding.
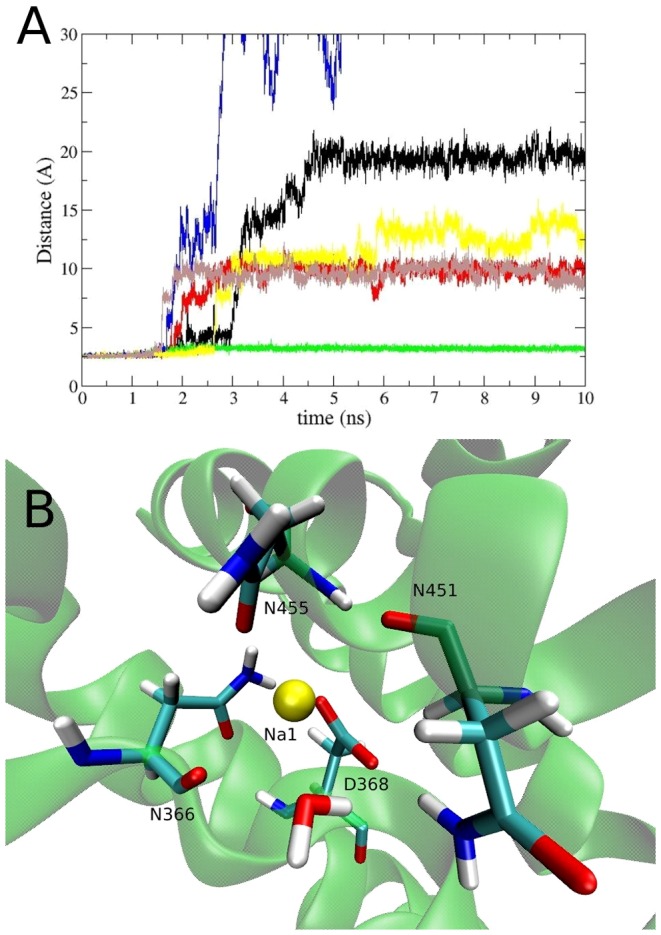
(A) The distance between the Na1 ion and the side-chain carboxyl/carboxamide carbon atom of the residue D455 during its mutation from aspartate to an asparagine (black, red and green), and to a protonated aspartate (blue, yellow and brown) in the presence of the Na3 ion. In the first case, Na1 leaves the binding site in 2 out of 3 chains, and in the second case, Na1 leaves the binding site in all chains. (B) The new coordination of Na1 when the D455N mutation is performed in the absence of the Na3 ion. This site has the coordination of the carbonyl oxygens of N366 and N451, as well as the side chain oxygens of D368 and N455.
