Table 1. Effect of different mutations on the properties of EAAT1, EAAT2 (GLT-1) and EAAT3 (EAAC1) transporters.
| Transporter | Trans. | Exch. | Na+ aff.(s) | Na+ aff. | K+ int. | Li+ int. | Glu aff.* | Asp aff.* | |
| EAAT1 | Wild-Type [4], [5] | Yes | Yes | 20 mM | Yes | Yes | 24 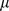 M M |
16 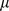 M M |
|
| T131A [6] | Yes | N/S | 1Red | ||||||
| S363R/R477M [4] | No | Yes | 0Inc | No | 2Inc | 2Inc | |||
| EAAT2 | Wild-Type [7]–[10] | Yes | Yes | 24 mM | Yes | No | 5.3 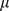 M M |
2.8 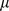 M† M†
|
|
| Y403F [9] | No | Yes | 1Inc | No | Yes | WT | WT† | ||
| E404D [7] | No | Yes | WT | No | |||||
| S440G [10] | Yes | Yes | Yes | Yes | WT | WT† | |||
| EAAT3 | Wild-Type [11]–[13] | Yes | Yes | 8 mM | 120 mM | Yes | Yes | 14 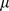 M M |
14 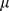 M M |
| T101A [14] | Yes | Yes | 0Red | N/S | Yes | 2Red | |||
| H296Q [15] | Yes | Yes | WT | Yes | WT | ||||
| N366Q [16] | Yes | Yes | N/S | Yes | No | 3Red | 2Red | ||
| D368E [16] | Yes | Yes | WT | Yes | No | None | 1Red | ||
| D368N [13] | No | Yes | 0Red | N/S | No | 2Red | |||
| N366D/D368N [16] | No | Yes | No | No | 1Red | 0Red | |||
| T370S [12], [17], [18] | Yes | Yes | N/S | 0Red | Yes | No | 2Red | WT | |
| E374Q [11], [19] | Yes | Yes | WT | WT | Yes | WT | |||
| D440N [11] | No | No | 1Red | WT | 2Red | 1Red | |||
| D440E [20] | Yes | Yes | 1Inc | Yes | Yes | 1Inc | 1Inc | ||
| D444C [21] | No | Yes‡ | WT | WT | |||||
| D444E [21] | No | Yes | 0Inc | WT | WT | ||||
| R445E [21] | Yes | Yes | |||||||
| R445S [22] | Yes | Yes | WT | Yes | 1Inc | 1Inc† | |||
| R447C [23] | No | No | Becomes a Na+ dependent serine/cysteine exchanger | ||||||
| N451S [17] | Yes | Yes | N/S | Yes | No | 2Red | 1Red | ||
| D455N [11], [13], [24], [25] | No | Yes | WT | WT | Yes | Yes | 0Inc | 0Inc | |
| D455S [24] | No | Yes | 0Red | No | Yes | WT | 0Inc | ||
| D455E [24] | No | No | |||||||
| D455A [24]–[26] | No | No | WT | ||||||
Transporter/Exchanger functionality is given in the third and fourth columns. Columns 5 and 6 gives the Na+ affinity in the presence and absence of glutamate, respectively. Columns 7 and 8 specify K+ and Li+ interactions with the transporter. Glu and Asp affinities are given in the last two columns. For the affinities, N/S means no saturation of the binding site in the range considered, and nRed/nInc means reduction/increase in the ligand affinity by  orders of magnitude (0, same order). Human sequences are used for EAAT3 which have one extra residue than the rat sequences employed in some experiments.
orders of magnitude (0, same order). Human sequences are used for EAAT3 which have one extra residue than the rat sequences employed in some experiments.
*All glutamate affinities measured at [Na+] between 100–150 mM.
Result for D-aspartate.
Enables succinate exchange, unlike wild-type.
