Figure-4.
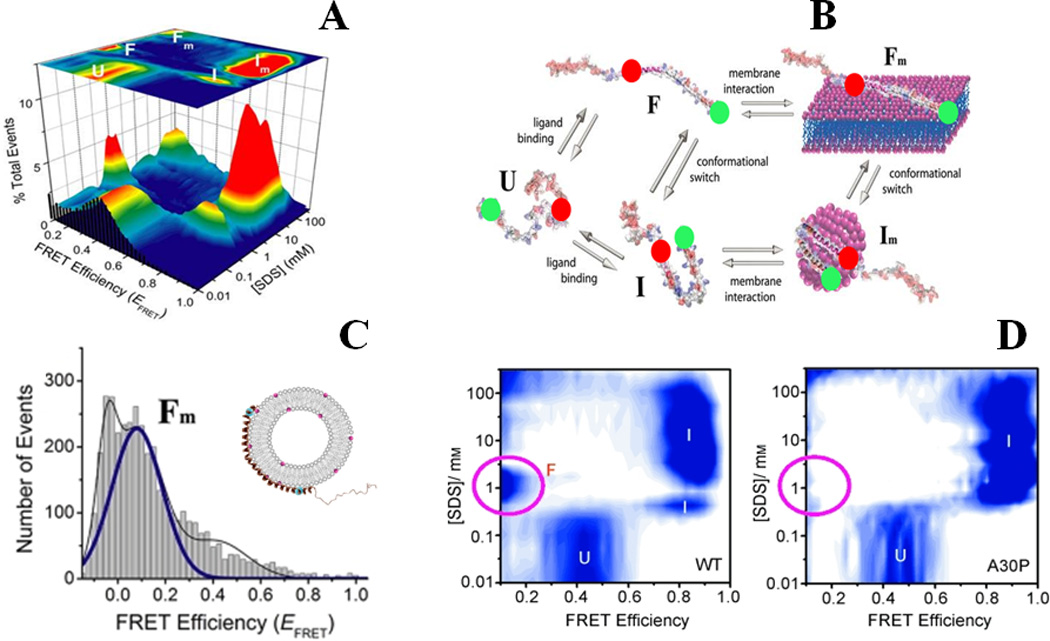
Binding-coupled-folding of α-synuclein: (A) and (B) show multistate conformational sampling of synuclein as revealed during SDS-binding of the protein probed by smFRET studies. smFRET histograms are shown in (A), and a schematic describing different states of α-synuclein is shown in (B). Red and green spheres in (B) represent donor and acceptor dyes. (C) Shows an extended helical conformation, the Fm state, when the protein is bound to phospholipid SUVs (POPA:POPC = 1:1). The disease-linked A30P mutation alters the protein-folding landscape substantially, as observed in (D) by a largely absent F state. (A), (B) and (C) are adapted from Ferreon et al., Proc. Natl. Acad. Sci. (2009) 106:5645 21, and (D) is adapted from Ferreon, Moran et al., Angew. Chem. Int. Ed. (2010) 49:3469 25.
