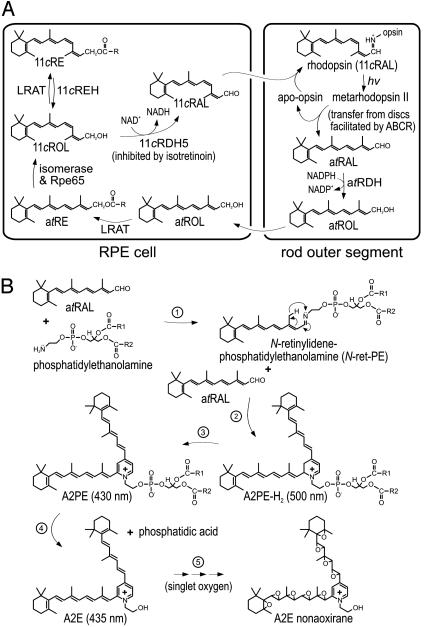
Fig. 1.
Retinoid pathways in the retina and RPE. (A) Visual cycle mediating rhodopsin regeneration. Absorption of a photon (hv) by a rhodopsin molecule in a rod outer segment disk induces photoisomerization of the 11cRAL chromophore, yielding activated metarhodopsin II. After several seconds, metarhodopsin II decays to yield apo-rhodopsin and free atRAL. ABCR functions to accelerate removal of atRAL from the interior of outer segment discs to the cytoplasmic space by flipping N-ret-PE (5). The atRAL is subsequently reduced to atROL or vitamin A by all-trans-retinol dehydrogenase. The atROL is released from the outer segment and taken up by an adjacent RPE cell where it is esterified by lecithin retinol acyl transferase (LRAT) to form an atRE. Chemical isomerization is effected by an isomerase that uses atREs as a substrate, in conjunction with Rpe65 (46). The resulting 11cROL is oxidized by 11cRDH to form 11cRAL chromophore. 11cRDH is inhibited by isotretinoin with a Ki of ≈0.1 μM (40, 47). 11cROL may also serve as a substrate for LRAT to form 11-cis-retinyl esters. The final step is recombination of 11cRAL with aporhodopsin in the outer segment to form a new molecule of light-sensitive rhodopsin. (B) Synthesis of A2E. After light exposure, newly released atRAL condenses reversibly with phosphatidylethanolamine to N-ret-PE (step 1). Rarely, a second molecule of atRAL will condense with N-ret-PE to form A2PE-H2 (step 2). The wavelength of maximal absorption (λmax) for A2PE-H2 is 500 nm. Within the acidic and oxidizing environment of RPE phagolysosomes, A2PE-H2 is oxidized to A2PE (λmax = 430 nm) (step 3). Hydrolysis of the phosphate ester yields A2E (λmax = 435 nm) and phosphatidic acid (step 4) (10). Double bonds along the polyene chains of A2E may react with singlet oxygen to form a series of one to nine (shown) oxiranes (step 5).