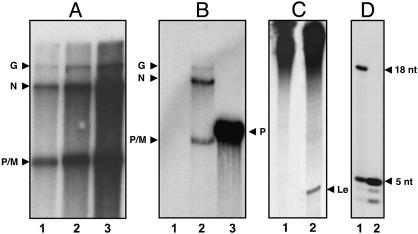
Fig. 2.
Characterization of transcriptase. (A) In vitro RNA synthesis was carried out by purified transcriptase fraction by using [α-32P]GTP as the labeled precursor as described in Materials and Methods. The RNA products were analyzed in 5% urea-PAGE. Lanes 1 (500 ng), 2 (1 μg), and 3 (2 μg). (B) Capping status of the transcriptase fraction from anti-P eluate was analyzed by capping the unlabeled RNA products with vaccinia GT and [α-32P]GTP (lane 1). The unlabeled RNA products were decapped with tobacco acid pyrophosphatase followed by dephosphorylation and end-labeling RNA products with [γ-32P]ATP and polynucleotide kinase (lane 2). As a control, the T7 transcript of VSV P was capped with vaccinia capping enzyme (lane 3). The transcripts were visualized by electrophoresis in 5% urea-PAGE followed by autoradiography. (C) Leader RNA synthesis. In vitro transcription reaction was carried out by using transcriptase fraction (500 ng) from anti-P eluate (lane 1) or RNP (300 μg) (lane 2), and the products were analyzed in 20% urea-PAGE. The position of the leader is marked as Le. (D) Oligonucleotide synthesis by the transcriptase in the absence of UTP. A standard in vitro transcription reaction was carried out in a 50-μl reaction containing 0.5 mM ATP, 150 μM CTP, 150 μM GTP, and 20 μCi of 6,000 Ci/mmol CTP, with virus (20 μg) (lane 1) or transcriptase (5 μg) (lane 2), and incubated at 30°C for 2 h. The reaction products were purified by phenol extraction followed by calf intestinal alkaline phosphatase treatment and analyzed in 20% urea-PAGE.