Abstract
Chlamydia trachomatis and C. muridarum are human and mouse pathogens respectively, which show high conservation of gene order and content. Both species contain a common 7.5 kb plasmid that is an important virulence factor. Recently described transformation systems have been used to characterize C. trachomatis L2 plasmid gene functions; however, similar studies have not been reported for C. trachomatis ocular tropic serovar A or the mouse strain, C. muridarum. Here, we have conducted genetic experiments with C. trachomatis serovar A and C. muridarum and report the following; (i) successful transformation of C. muridarum and C. trachomatis serovar A is restricted to a shuttle vector with a C. muridarum, or C. trachomatis serovar A plasmid backbone, respectively, ii) transformation of plasmid-deficient C. muridarum with the C. muridarum based shuttle vector complemented glycogen accumulation and inclusion morphology, and (iii) C. muridarum plasmid encoded Pgp4 is a regulator of chromosomal (glgA) and plasmid (pgp3) virulence genes. In summary, our findings show a previously unrecognized and unexpected role for the chlamydial plasmid in its transformation tropism and confirm the plasmids regulatory role of virulence genes in C. muridarum.
Keywords: Chlamydiae, plasmid, transformation, tropism
Chlamydia trachomatis is an obligate intracellular human pathogen with a unique biphasic developmental growth cycle (Moulder, 1966). It is the etiological agent of trachoma, the world’s leading cause of preventable blindness and the most common cause of bacterial sexually transmitted disease (Schachter, 1978; Whitcher et al, 2001; Brunham & Rey-Ladino, 2005). C. trachomatis infection tropism is largely restricted to humans and nonhuman primates (NHP); a characteristic that has hampered progress in the understanding of C. trachomatis pathogenesis, immunity, and vaccine development. C. muridarum, a mouse pathogen which shows high conservation of gene order and content to C. trachomatis, is commonly used as a model of human C. trachomatis reproductive tract infection (Morrison et al, 2000; Morrison & Caldwell, 2002; Lyons et al, 2009; Farris & Morrison, 2011; Shao et al, 2012). C. trachomatis and C. muridarum isolates carry a highly conserved 7.5 kb dsDNA plasmid that is an important virulence factor in NHP and murine infection models, respectively (O'Connell, et al 2007; Kari, et al 2011). It has been shown for both C. trachomatis and C. muridarum that the plasmid regulates the transcription of multiple chlamydial chromosomal genes, designated plasmid-responsive chromosomal loci (PRCL) (Carlson et al, 2008; O'Connell et al, 2011). One of the plasmid-regulated loci is glgA, encoding for the enzyme glycogen synthase. Regulation of glycogen synthase expression explains the glycogen negative phenotype displayed by naturally occurring and laboratory generated plasmid-deficient C. trachomatis isolates (Carlson et al, 2008). Moreover, the C. trachomatis plasmid has been shown to regulate the expression of pgp3 (Song et al, 2013), the plasmid gene that encodes for the secreted virulence factor Pgp3 (Li et al, 2008). The plasmids of C. muridarum and C. trachomatis encode eight highly conserved open reading frames (ORFs) (Stephens et al, 1998; Read et al, 2000). Using the recently described plasmid-based method for transforming C. trachomatis L2 (Wang et al, 2011), we employed a PCR based mutagenesis procedure to make deletion mutations in all 8 L2 plasmid ORFs (Song et al, 2013). The studies showed that Pgp4 was a transcriptional regulator of both plasmid encoded pgp3 and the numerous PRCLs (Gong et al 2013; Song et al, 2013).
A significant limitation to using C. trachomatis L2 for genetic studies is that there is no relevant animal model mimicking human disease for the LGV serovars. The purpose of this study was to develop a transformation system for C. muridarum and the non-LGV C. trachomatis trachoma isolate (strain A2497) which would then allow us to generate specific plasmid ORF deletion mutants that could be used to define the pathogenic role(s) of individual plasmid genes in relevant murine and NHP animal models, respectively.
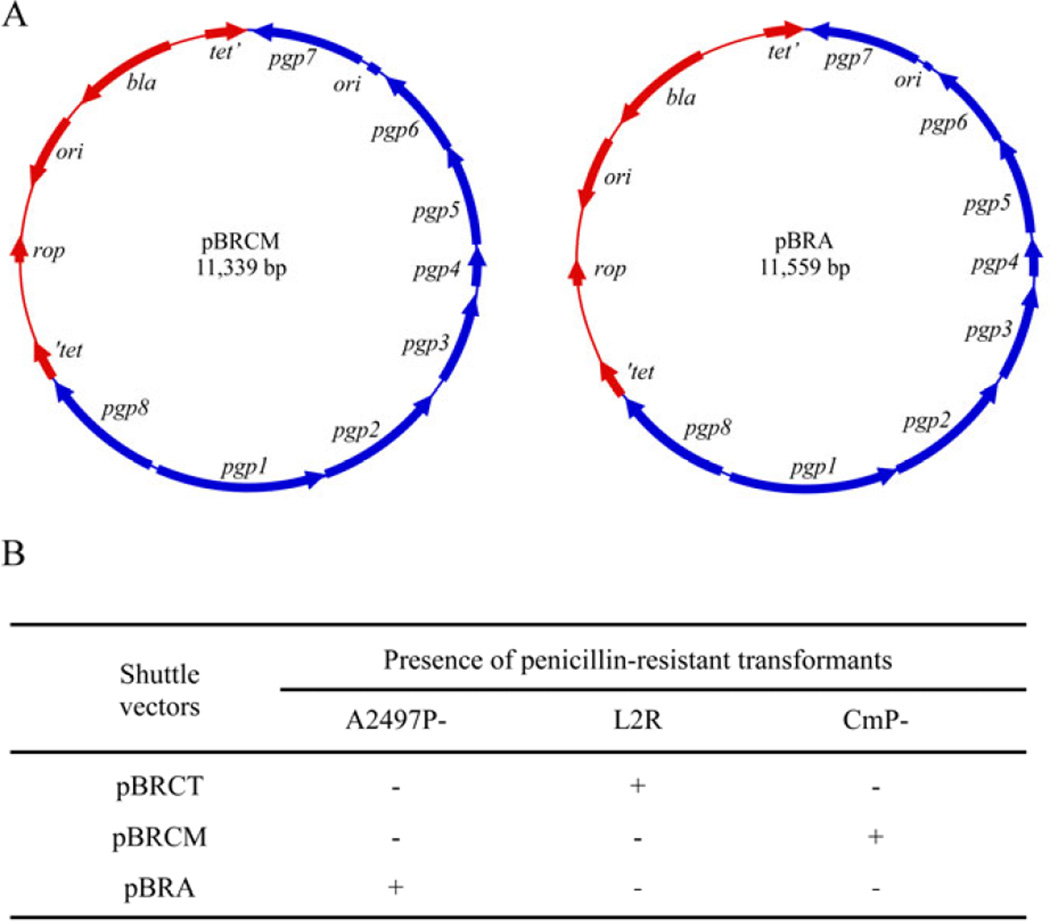
Plasmid-deficient C. muridarum and C. trachomatis serovar A (strain A2497), designated CmP− and A2497P−, respectively were isolated employing novobiocin curing as previously described (O'Connell & Nicks, 2006; Kari et al, 2011). The endogenous shuttle vectors pBRCM for C. muridarum and pBRA for C. trachomatis A2497 were constructed similarly to our L2 based shuttle vector pBRCT (Song et al, 2013) with a pBR322 backbone and all eight plasmid ORFs intact (Fig 1A). The results from transformation experiments utilizing plasmidless chlamydial strains L2R, CmP−, A2497P− with homologous and heterologous plasmid shuttle vectors are shown in Figure 1B. Remarkably, stable penicillin G resistant transformants of L2R, CmP−, and pBRA were only isolated if the shuttle vector employed for transformation had the chlamydial plasmid backbone from its matched parental strain.
Fig. 1.
Construction of chlamydial plasmid shuttle vectors and homotypic plasmid transformation tropism. (A) C. muridarum pBRCM and C. trachomatis A2497 pBRA. pBRCM was constructed using primers JHC626 (5’-GGCCGGCGGCCGCCCTCAAAAGCAACTGTAGATTATATTAGGGCCATC-3’) and JHC627 (5’-CCGGCCCGCCGGTGAACTAAATGGATATAATTTTAATTATATCACAATATAGT TGG-3’), introducing EagI and SgrAI sites respectively. pBRA was constructed using primers JHC740 (5’-GGCCGGCGGCCGTGTTGCCAGAAAAAACACCTTTAGGCTATATTAGAG-3’) and JHC741 (5’-GGCCGGGTCGACGAATATGAATATAATTTTAATTATATCACAATATTG-3’), introducing EagI and SalI sites respectively. Amplification using Phusion High-Fidelity DNA polymerase kit (New England Biolabs) and insertion into pBR322 was conducted as previously described (Song et al, 2013). (B) Transformation tropism of different chlamydial plasmid shuttle vectors. The results are representative of 3 independent experiments. Stable transformation is defined as a minimum of 3 successive passages of transformants grown in the presence of penicillin G. Transformation of CmP− was conducted as previously described (Song et al, 2013) while transformation of A2497P− followed the same protocol with minor modifications. The modifications include; (1) changing to media containing 10 U/ml PenG at 20 h post-infection and (2) selecting transformants in six well plates by using centrifugation-aided infection.
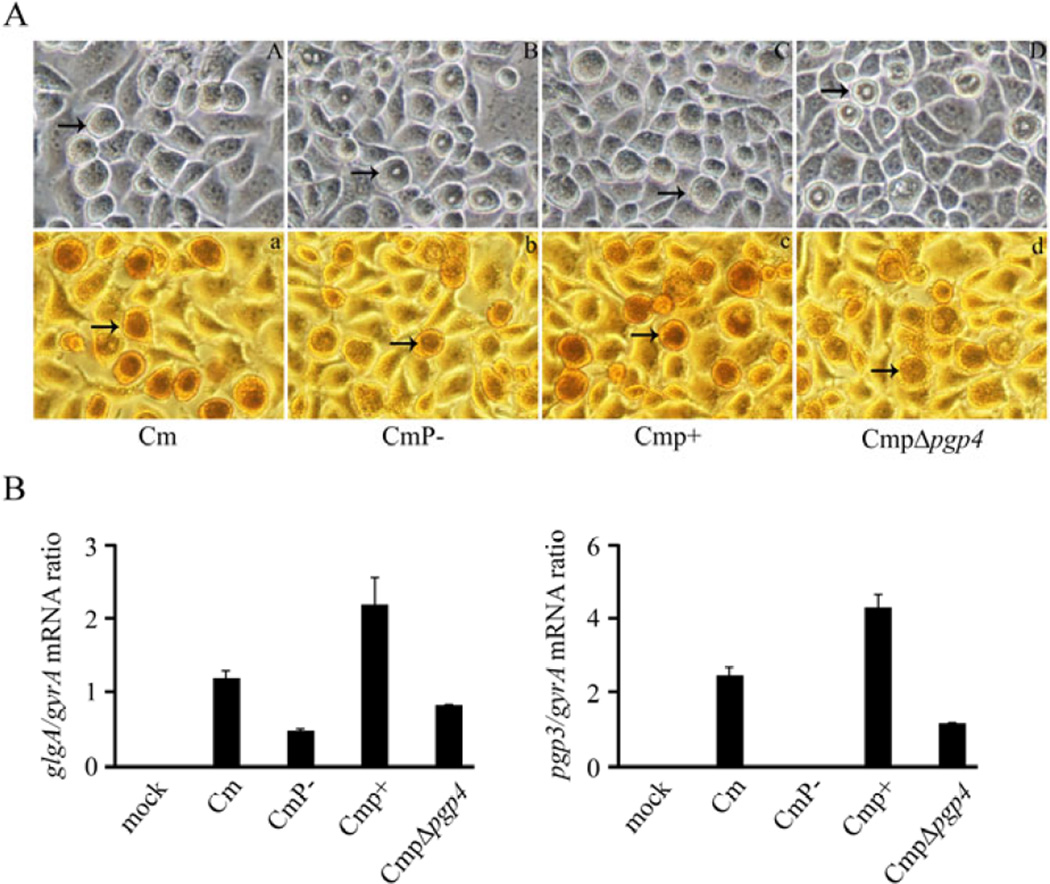
Chlamydial plasmid-deficient organisms exhibit characteristic atypical late-inclusion morphology with a donut appearance that fails to stain or stains weakly for glycogen (Matsumoto et al, 1998; O'Connell & Nicks, 2006; Carlson et al, 2008; Wang et al, 2013b). Consistent with these characteristics, late-infection inclusions of CmP− displayed donut-like morphology and showed weaker iodine staining when compared to wild type plasmid-bearing C. muridarum (Fig.2A). Both normal inclusion morphology and stronger iodine staining were restored in CmP− following transformation with pBRCM (Cmp+).
Fig. 2.
Glycogen staining and qRT-PCR analysis of C. muridarum plasmid gene mutants. (A) Phase microscopy and iodine staining of McCoy cells infected with Cm, CmP−, Cmp+ and CmpΔpgp4. Cells were infected with a MOI of 0.25 and examined at 30 h post-infection (magnification 200×). Arrows indicate inclusions. (A, a) Cm. (B, b) CmP−. (C, c) CmP+. (D, d) CmpΔpgp4. The inclusions of CmP− and CmpΔpgp4 exhibit a donut-like appearance and exhibited reduced glycogen staining. (B) glgA and pgp3 transcript levels in C. muridarum strains. The differences in glgA expression is significantly different (p<0.05) in CmP− vs Cm and CmpΔpgp4 vs Cmp+ comparisons. The differences in pgp3 expression is significantly different (p<0.05) in CmP− vs Cm and CmpΔpgp4 vs Cmp+ comparisons.
We (Song et al, 2013), and subsequently others (Gong et al, 2013; Wang et al, 2013a), have shown transformed C. trachomatis L2 organisms with a disrupted pgp4 (L2RpΔpgp4) produced the same inclusion and glycogen staining phenotypes as plasmid-deficient organisms. To determine if C. muridarum Pgp4 conferred the same phenotypic characteristics to chlamydial inclusions we constructed pBRCMΔpgp4. McCoy cells infected with CmpΔpgp4 (CmP− transformed with pBRCMΔpgp4) revealed inclusions identical to CmP− with similar donut-like inclusion morphology and weaker iodine staining (Fig. 2A). The less intense glycogen staining by CmP− and CmpΔpgp4 is however different from our previous finding with L2R and L2RpΔpgp4, where both organisms gave rise to inclusions that were negative for glycogen staining (Song et al, 2013).
In our previous studies we showed that the C. trachomatis L2 plasmid is a transcriptional regulator of both plasmid (pgp3) and numerous chromosomal genes, including the gene encoding for glycogen synthase, glgA. We further showed using the L2 transformant L2RpΔpgp4 that pgp4 alone is responsible for the transcriptional regulatory function of the plasmid. To determine if C. muridarum pgp4 possessed a similar regulatory function the pgp4 deletion mutant (CmpΔpgp4) was characterized transcriptionally. qPCR showed that cells infected with CmpΔpgp4 had significantly lower transcript levels of both glgA and pgp3 (Fig. 2B) than the parental plasmid-bearing C. muridarum. There was however less reduction of glgA transcript between CmP−/CmpΔpgp4 vs. L2R/ L2RpΔpgp4 organisms (Song et al, 2013) a finding consistent with our observation that CmP− and CmpΔpgp4 retained weak glycogen staining (Fig 1B).
The ability to transform chlamydiae (Wang et al, 2011) has allowed for genetic studies of chlamydial plasmid gene functions and initial insights into chlamydial virulence characteristics (Gong et al, 2013; Song et al, 2013). However, only a limited number of chlamydial strains have been successfully transformed (Wang et al, 2011; Song et al, 2013; Wang et al, 2013b) and those that have lack practical small animal or NHP models for the study of chlamydial pathogenesis. To circumvent this shortcoming, we have extended the plasmid based transformation system to include C. muridarum and C. trachomatis serovar A strains with well accepted mouse and NHP animal models, respectively.
Our results show a strong transforming tropism associated with the plasmid. Stable transformants were obtained only when transforming plasmid shuttle vector and host were matched. In contrast, previous studies have shown that the chlamydial shuttle vector pGFP::SW2 can be used to transform L2, the naturally occurring plasmid-deficient strain L2R and a plasmid-deficient serovar F strain (Wang et al, 2011; Gong et al, 2013; Wang et al, 2013b). While we are unsure of the molecular basis which allows pGFP::SW2 to be more promiscuous for chlamydial transformation there are clear differences between this construct and our shuttle vectors. pGFP::SW2 was constructed using the backbone of the endogenous plasmid isolated from the Swedish new variant serovar E (Wang et al, 2011). This plasmid carries a 377 bp deletion within pgp7, which covers the single target originally used by Roche and Abbott diagnostic systems (Unemo & Clarke, 2011). In contrast, pBRA and pBRCM used in this study have intact pgp7 ORFs. In addition, the Swedish new variant endogenous plasmid, and thus also pGFP::SW2, carries a 44 bp duplication immediately upstream of both pgp8 and pgp1. At this time we do not know if this duplication effects the expression of pgp1 and/or pgp8, which are divergently transcribed. Perhaps a transformation tropism exists between LGV and trachoma biovars but not genital and LGV biovars. In support of this hypothesis, we have not been able to isolate stable transformants of plasmid-deficient A2497 using pBRCT or transformants of plasmid-deficient L2R using pBRA. We are precluded from trying to transform plasmid cured non-LGV genital strains in the United States as penicillin is used to treat chlamydial infections in pregnant women, thus the experiments cannot receive NIH approval.
Phylogenetic analysis of chlamydial genomes and plasmids has implicated a co-evolutionary relationship and the fact that the plasmid does not naturally transfer readily between clinical isolates support a host-plasmid tropism relationship (Seth-Smith et al, 2009). Most strikingly, despite rapid and wide transmission in Sweden, the Swedish new variant plasmid has only turned up in a serovar E background (Jurstrand et al, 2010). This suggests that despite strong diagnostic selective advantage the transfer of the new variant plasmid to other genital serovars, which were undoubtedly circulating in the population, has occurred infrequently, if at all. Our experimental findings of plasmid tropism among chlamydial strains further support this hypothesis.
We show that similar to the C. trachomatis plasmid the C. muridarum plasmid also transcriptionally regulates glgA and pgp3. However; C. muridarum plasmid-mediated regulation of glgA appears to be less stringent than in C. trachomatis. These subtle but perhaps important transcriptional differences between human and mouse strains were not evident for the plasmid encoded pgp3. The potential significance of this observation is unknown, but it suggests that differences in plasmid and chromosomal transcriptional control mechanisms of glycogen biosynthesis between mouse and human strains might exist that could impact the relative attenuation phenotype of the plasmidless strains in their respective hosts.
ACKNOWLEDGEMENTS
We thank Kelly Matteson and Anita Mora for editorial and graphic assistance. Kayley Schulmeyer for assistance with the qRT-PCR analyses. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 2.Carlson JH, Whitmire WM, Crane DD, et al. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong S, Yang Z, Lei L, Shen L, Zhong G. Characterization of Chlamydia trachomatis plasmid-encoded open reading frames. J Bacteriol. 2013;195:3819–3826. doi: 10.1128/JB.00511-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurstrand M, Christerson L, Klint M, Fredlund H, Unemo M, Herrmann B. Characterisation of Chlamydia trachomatis by ompA sequencing and multilocus sequence typing in a Swedish county before and after identification of the new variant. Sex Transm Infect. 2010;86:56–60. doi: 10.1136/sti.2009.037572. [DOI] [PubMed] [Google Scholar]
- 6.Kari L, Whitmire WM, Olivares-Zavaleta N, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. 2011;208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons JM, Igietseme JU, Black CM, Morre SA. Identification of candidate genes using the murine model of female genital tract infection with Chlamydia trachomatis. Drugs Today (Barc) 2009;45(Suppl B):51–59. [PubMed] [Google Scholar]
- 9.Matsumoto A, Izutsu H, Miyashita N, Ohuchi M. Plaque formation by and plaque cloning of Chlamydia trachomatis biovar trachoma. J Clin Microbiol. 1998;36:3013–3019. doi: 10.1128/jcm.36.10.3013-3019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulder JW. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- 13.O'Connell CM, Nicks KM. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology. 2006;152:1601–1607. doi: 10.1099/mic.0.28658-0. [DOI] [PubMed] [Google Scholar]
- 14.O'Connell CM, Ingalls RR, Andrews CWJ, Scurlock AM, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;176:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 15.O'Connell CM, AbdelRahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun. 2011;79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read TD, Brunham RC, Shen C, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schachter J. Chlamydial infections (first of three parts) N Engl J Med. 1978;298:428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 18.Seth-Smith HM, Harris SR, Persson K, et al. Co-evolution of genomes and plasmids within Chlamydia trachomatis and the emergence in Sweden of a new variant strain. BMC Genomics. 2009;10:239. doi: 10.1186/1471-2164-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao R, Wang X, Wang W, Stener-Victorin E, Mallard C, Brannstrom M, Billig H. From mice to women and back again: Causalities and clues for Chlamydia-induced tubal ectopic pregnancy. Fertil Steril. 2012;98:1175–1185. doi: 10.1016/j.fertnstert.2012.07.1113. [DOI] [PubMed] [Google Scholar]
- 20.Song L, Carlson JH, Whitmire WM, et al. Chlamydia trachomatis Plasmid-Encoded Pgp4 Is a Transcriptional Regulator of Virulence-Associated Genes. Infect Immun. 2013;81:636–644. doi: 10.1128/IAI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens RS, Kalman S, Lammel C, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 22.Unemo M, Clarke IN. The Swedish new variant of Chlamydia trachomatis. Curr Opin Infect Dis. 2011;24:62–69. doi: 10.1097/QCO.0b013e32834204d5. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Cutcliffe LT, Skilton RJ, Persson K, Bjartling C, Clarke IN. Transformation of a plasmid-free, genital tract isolate of Chlamydia trachomatis with a plasmid vector carrying a deletion in CDS6 revealed that this gene regulates inclusion phenotype. Pathogens and Disease. 2013a;67:100–103. doi: 10.1111/2049-632X.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Persson K, Bjartling C, Clarke IN. Genetic Transformation of a Clinical (Genital Tract), Plasmid-Free Isolate of Chlamydia trachomatis: Engineering the Plasmid as a Cloning Vector. PLoS One. 2013b;8:e59195. doi: 10.1371/journal.pone.0059195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull WHO. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]