Abstract
Health disparities research seeks to eliminate disproportionate negative health outcomes experienced in some racial/ethnic minority groups. This brief review presents findings on factors associated with drinking and alcohol-related problems in racial/ethnic groups. Those discussed are: 1) biological pathways to alcohol problems, 2) gene by stress interactions, 3) neighborhood disadvantage, stress, and access to alcohol, and 4) drinking cultures and contexts. These factors and their interrelationships are complex, requiring a multi-level perspective. The use of interdisciplinary teams and an epigenetic focus are suggested to move the research forward. The application of multi-level research to policy, prevention, and intervention programs may help prioritize combinations of the most promising intervention targets.
Keywords: Alcohol, Race, Ethnicity, Health Disparities, Associated Factors
Epidemiological studies show variations across and within the U.S. in drinking patterns, alcohol use disorders (AUDs), and associated negative consequences (Chartier and Caetano, 2010), including for the four largest racial/ethnic groups in the U.S. (Whites, Blacks, Hispanics, and Asians). Whites have a greater risk for AUDs relative to other racial/ethnic groups. Compared to Whites, high rates of heavy drinking are reported for Hispanic drinkers and lower rates for Asian drinkers, although subgroup differences are evident. Compared to Whites and Asians, Hispanics and Blacks are disproportionately disadvantaged by health and social problems from drinking.
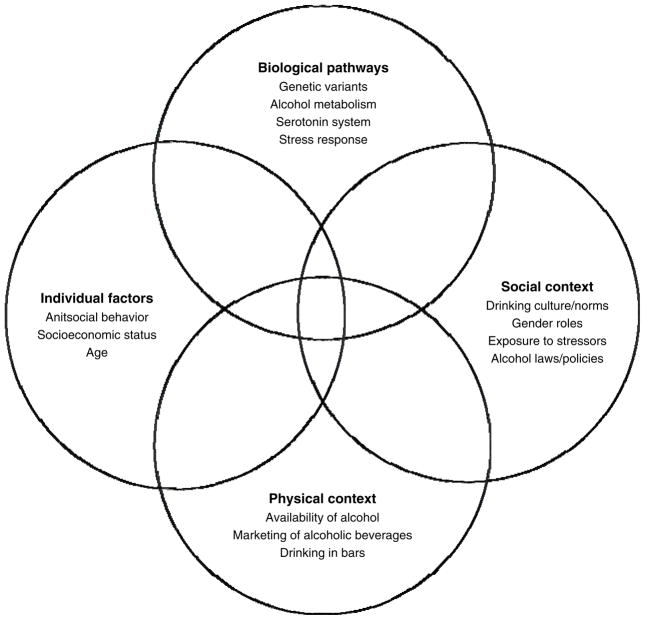
This brief review aims to provide a multi-level perspective (see Figure 1) on the factors that may affect within racial/ethnic group drinking behaviors as well as differences between groups in drinking and alcohol-related problems. It serves to illustrate areas in which more complex research on racial/ethnic variations in drinking and alcohol-related problems is needed. The following factors are highlighted based on a symposium at the 2012 scientific meeting of the Research Society on Alcoholism: 1) biological pathways to alcohol problems, 2) gene by stress interactions, 3) neighborhood disadvantage, stress, and access to alcohol, and 4) drinking cultures and contexts.
Figure 1.
Factors associated with racial/ethnic disparities in drinking and alcohol outcomes
Racial/ethnic groups in this review may be alternately referred to as White or European American, Black or African American, Hispanic or Latino, and Asian or Asian American. The use of race/ethnicity categories oversimplifies the diversity of people and the difference in drinking patterns observed within these groups, but nevertheless helps to identify high-risk groups. Conceptual models for health disparities point to the importance of factors from biology to macro social conditions in understanding disparities in disease burden (Warnecke et al., 2008). Understanding the complex determinants of alcohol outcomes for racial/ethnic minority groups will enable researchers to develop better, more targeted interventions to reduce and ultimately eliminate disparities in alcohol-related outcomes.
ETHNIC VARIATIONS IN BIOLOGICAL PATHWAYS TO ALCOHOL PROBLEMS
Epidemiological studies in the U.S. indicate Asians have lower rates of AUDs than Whites, Blacks, and Hispanics (Hasin et al., 2007). However, when Asians are disaggregated, there are substantial subgroup differences in rates of AUDs both across and within national groups (Helzer et al., 1990, Luczak et al., 2004). These differences are hypothesized to result from variations in both genetic and environmental risk and protective factors. We review genetic factors that are known to vary in their prevalence across ethnic groups (particularly Asian subgroups), and then examine additional risk and protective factors that may add to and/or interact with genetic determinants to contribute to differences in AUD prevalence across ethnic groups.
The genes with the strongest association with alcohol dependence are those involved in alcohol metabolism, specifically alcohol and aldehyde dehydrogenase genes ADH1B and ALDH2 (see Hurley and Edenberg, 2012). Two ADH1B variant alleles, ADH1B*2 and ADH1B*3, encode for enzymes that increase the rate of metabolism of alcohol into acetaldehyde. The variant ALDH2*2 allele encodes enzymes that decease the rate of conversion of acetaldehyde into acetate. All three of these variant alleles theoretically increase the concentration of acetaldehyde during alcohol metabolism, resulting in heightened reactions to alcohol, e.g., flushing response in some people, which are hypothesized to reduce the likelihood of heavy drinking and the development of AUDs (Wall, 2005).
ADH1B*2 is well established as a protective factor against AUDs in Asians (Luczak et al., 2006) and more recent large-scale studies and meta-analyses indicate a similar protective effect in Europeans (Hurley and Edenberg, 2012). ADH1B*2 is found in 80–95% of northeast Asians, 50% of Ashkenazi Jews, but less than 10% of western Europeans. ADH1B*3 is found primarily in individuals of Western African descent (15–30%), and in low prevalence in Native-Americans (6%), with protective effects in both samples (Wall et al., 2003, Hurley and Edenberg, 2012).
The strongest genetic association with AUDs has been reported for ALDH2*2 allele (Hurley and Edenberg, 2012). ALDH2*2 is found almost exclusively in Asians, with rates being 30–50% for Chinese, Koreans, and Japanese (Eng et al., 2007). A meta-analysis found possessing one ALDH2*2 allele related to a four-to-five-fold reduction in alcohol dependence, and two ALDH2*2 alleles with an eight-to-nine-fold reduction (Luczak et al., 2006). Protection against AUDs afforded by ALDH2*2 is strong, but the strength of this association appears to be modified by other variables. Because of the large protective effect of ALDH2*2 and the high prevalence of this allele in northeast Asians, it is possible to examine how this gene operates in conjunction with other risk and protective factors for AUDs (e.g., drinking culture, gender roles, antisocial behavior) both within and between Asian subgroups.
The first demonstration of an interaction of ALDH2*2 was reported by Higuchi and colleagues (1994) in Japan. They found rates of Japanese with ALDH2*2 in alcohol treatment increased from 3% in 1979, to 8% in 1986, to 13% in 1992. During this time, per capita alcohol use in Japan also increased. This suggests that increased prevalence of alcohol use and social pressure to drink reduced the effect of ALDH2*2 on drinking behavior over time.
Studies in Asia and the U.S. have consistently found higher rates of alcohol dependence in individuals of South Korean heritage compared with Taiwanese and Chinese heritage (Helzer et al., 1990, Luczak et al., 2004). Different rates of alcohol dependence between Koreans and Chinese are partly explained by ALDH2*2 prevalence, with approximately 30% of Koreans and up to 50% of Chinese possessing ALDH2*2 (Eng et al., 2007), but data also suggest the strength of the ALDH2*2 effect may differ across these groups (Luczak et al., 2004, 2006). Furthermore, the prevalence of AUDs, appears to be decreasing in the country of South Korea and increasing in China (Cochrane et al., 2003, Hahm and Cho, 2005, Helzer et al., 1990). It has been suggested that the AUD patterns in South Korea and China have become more similar to those in the U.S. over the past 20 years, and these changes have been attributed to shifts in sociocultural influences and westernization. For example, as the social pressures against drinking are relaxed for Asian women (Cochrane et al., 2003, Hahm and Cho, 2005), the genetic contribution of ALDH2*2 to women’s development of AUDs may also change. Gender differences in AUDs are particularly pronounced in many Asian national groups, despite similar prevalence of ALDH2*2 across gender. However, the male-to-female ratio of AUDs has decreased in recent decades both in South Korea and in China (Hahm and Cho, 2005, Cochrane et al., 2003, Helzer et al., 1990).
The protection of ALDH2*2 against AUDs may also be modified by within-person risk factors. Conduct disorder and antisocial personality disorder, which are consistently associated with AUDs, are found in higher prevalence in Korean ethnic groups compared with Taiwanese and Chinese ethnic groups (Hwu et al., 1989, Lee et al., 1990, Luczak et al., 2004). In a study examining ALDH2*2 and conduct disorder as explanatory variables for ethnic group differences in alcohol dependence prevalence, more Chinese Americans (52%) possessed an ALDH2*2 allele than Korean Americans (34%) (Luczak et al., 2004). Korean Americans had a higher rate of conduct disorder (15%) than European (9%) and Chinese (6%) Americans. Chinese Americans had a significantly lower rate of alcohol dependence (5%) than Korean (13%) and European (17%) Americans. The relationship of race/ethnicity to alcohol dependence was mediated by ALDH2 gene status and conduct disorder in an additive manner, indicating both ALDH2*2 and conduct disorder account for some the variability in rates of alcohol dependence among Korean, Chinese, and European Americans.
In summary, alcohol metabolizing genes variations are strongly associated with AUDs. These genetic factors interact and combine with social and individual factors to contribute to the different rates of AUDs found among racial/ethnic groups as illustrated here for Asians. Additional research is needed to examine these interrelationships across other racial/ethnic groups. This review further highlights the importance of disaggregating ethnic subgroups when studying the etiology of AUDs.
GENE X STRESS INTERACTION AND ALCOHOL CONSUMPTION
Studies examining gene by stress interactions and drinking may further inform research on racial/ethnic differences in alcohol outcomes. Two longstanding themes in alcohol research are the role of genetic variation and stress which has been associated with maladaptive alcohol use and relapse. While some genetic variants such as ALDH2*2 directly influence alcohol effects, emerging research suggests that genetic variation may also affect the risk of AUDs by moderating the influence of the social environment, neighborhood disadvantage, early life adversity, and recent negative life events on alcohol use. This line of research is relevant to understanding alcohol-related health disparities, as rates of post-traumatic stress disorder (PTSD), exposure to childhood violence, discrimination, and other adverse life stressors vary across racial/ethnic groups, with African Americans representing a particularly high risk group (Roberts et al., 2011). Work in this area has been informed by research on gene x environment effects on the risk of depression and PTSD (Caspi et al., 2010). Serotonin related genes and genes involved in mediating neuroendocrine stress responses have received the most focus as potential moderators of behavioral stress responses.
Individuals with lower levels of serotonin (5-HT) are thought to be at greater risk for developing stress-related disorders such as depression. Serotonin levels have also been linked to drinking behavior, with both animal and human studies indicating that lower levels of serotonin stimulate alcohol consumption (Sari et al., 2011). To the extent that serotonin is linked to both stress- and alcohol-related processes, genes that encode proteins involved in serotonin signaling represent potential genetic moderators of stress related drinking.
A widely studied candidate, SLC6A4, encodes the serotonin transporter protein (5-HTT) that regulates the magnitude and duration of serotonergic signaling. A tandem repeat polymorphism in the promoter region of the gene (5-HTTLPR) results in long (L) or short (S) alleles, the latter of which is associated with a reduction in 5-HTT mRNA and protein and a reduced functional coupling of the amygdala and medial prefrontal cortical areas that moderate amygdala activation. Research indicates that carriers of the S allele show stronger associations between depressive symptoms and both early life as well as more recent interpersonal, work, or medical stressors (reviewed in Caspi et al., 2010). In parallel with results related to depression are findings indicating that associations between life stress and drinking may also be moderated by 5-HTTLPR genotype. Researchers have found stronger associations between past year negative life events and drinking among S allele carriers, compared to L allele homozygotes, in both European American (Covault et al., 2007) and African American (Kranzler et al., 2012) college students. A growing body of work suggests that S allele carriers, while more sensitive to stress, may also be more responsive to treatment interventions to reduce impacts of environmental stressors (Brody et al., 2009).
Dysregulation of neuroendocrine stress response systems also is thought to be a risk factor for alcohol dependence and relapse during recovery (Sinha et al., 2011). Genes encoding proteins involved in regulation of neuroendocrine stress-response systems are natural candidates for gene x stress interactions and alcohol use. Studies examining the relationship of genetic variation in stress-response candidate genes to drinking and AUDs in multiple racial/ethnic groups and their interaction with specific types of stress are needed. Polymorphisms in genes involved in the activation and feedback regulation of the hypothalamic pituitary adrenal (HPA) axis (a major component of the neuroendocrine system) have been associated with stress-associated phenotypes including alcohol use. The corticotrophin releasing hormone receptor 1 (CRHR1) has a central role in HPA activation, and polymorphisms associated with the CRHR1 gene region have been reported to moderate associations between early life adversity and later alcohol use or depression in some but not all samples. In one large sample, interaction of CRHR1 gene variants and adverse childhood experiences on depression were only seen in African American women and not in European Americans or African American men (Kranzler et al., 2011). Interaction effects were strongest for childhood exposure to violent crime, followed by sexual or physical abuse.
The glucocorticoid receptor (GR) co-chaperone protein, FKBP5, which dampens GR response to cortisol, is being increasingly studied as a candidate for moderating stress-related outcomes. Polymorphisms in the FKBP5 gene have been associated with depression and PTSD in the setting of early life adversity (Binder, 2009), which may be more evident among alcohol involved African Americans compared with European Americans (Xie et al., 2010). Finally, a growing body of literature highlights polymorphisms in the gene encoding neuropeptide Y (NPY) as moderators of the neuroendocrine and behavioral responses to stress. NPY promoter polymorphisms in non-human primates are associated with lower levels of NPY protein in the amygdala and with higher arousal in response to acute stress and stress-reactive alcohol consumption (Lindell et al., 2010). In humans, lower expressing NPY genotypes are associated with increased amygdala and hippocampus activation to threat-related stimuli (Zhou et al., 2008) and cortisol response to a laboratory stressor in participants with a history of childhood adversity.
It will be important to examine the effects of polymorphisms in serotonin and stress response system candidate genes in conjunction with distal and recent adverse life stressors on drinking in multiple racial/ethnic populations. Results to date suggest that the effects of genetic variation in these genes on depression, PTSD, or drinking are only evident in the setting of environmental stress/adversity. Genetic effects strengthen with an increasing number of stressful events. Coping-related drinking motives (Cooper et al., 2008), the frequency of risk polymorphisms, and the types of environmental, social, and family stressors all appear to vary by racial/ethnic background.
NEIGHBORHOOD DISADVANTAGE, STRESS, AND ACCESS TO ALCOHOL
In addition to biological factors, risk factors in the macro environment vary widely across race/ethnicity in the U.S. Residential patterns depend on factors including choice, economic stratification, and segregation. As a result, African Americans, Hispanics, and Native Americans are significantly more likely than Whites to live in socioeconomically disadvantaged areas characterized by low incomes, low levels of education, unemployment, and working-class jobs. Residence in disadvantaged areas is often associated with increased substance use, even after adjusting for individual-level characteristics that influence neighborhood selection (Karriker-Jaffe, 2011). This association between area-level disadvantage and substance use emerges most robustly in the U.S. compared to other countries and appears strongest for heavy rather than light or moderate alcohol use, although results vary considerably across studies (Karriker-Jaffe, 2011).
Effects of neighborhood disadvantage on alcohol use and alcohol problems vary by race/ethnicity, with stronger effects for African Americans than Whites or Hispanics (Jones-Webb et al., 1997, Karriker-Jaffe et al., 2012). In a U.S. national sample of adult men, there were increased alcohol problems for African American (versus White) men in impoverished neighborhoods, with no differences between men in more affluent areas (Jones-Webb et al., 1997). Similarly, a U.S. national study of adult men and women showed differential impacts of neighborhood disadvantage for African Americans and Hispanics compared to Whites. Neighborhood disadvantage decreased heavy drinking by White drinkers, but increased heavy drinking by African American drinkers (and somewhat increased heavy drinking by Hispanic drinkers) (Karriker-Jaffe et al., 2012). Neighborhood disadvantage also increased negative alcohol-related consequences for African American men and White women, but not for White men, African American women, or Hispanics. Hispanics in the U.S. are more likely to live in disadvantaged neighborhoods with a high density of Hispanics, and these ethnic enclaves may decrease their risk of AUDs (Molina et al., 2012). Thus, while neighborhood context contributes to the patterning of alcohol outcomes by race/ethnicity, effects may be more nuanced for Hispanics.
Possible mechanisms by which neighborhood disadvantage increases racial/ethnic disparities in adverse alcohol use outcomes have been explored, as these neighborhoods pose many risks that may increase substance use. First, neighborhood disadvantage can increase residents’ stress, perhaps due to associations of disadvantage with crime and disorder, which may prompt some to drink heavily. Studies examining this neighborhood stress hypothesis are rare, but studies of individual-level disadvantage, distress, and drinking (Brown and Richman, 2012) and of neighborhood-level disadvantage, stress, and smoking by African Americans (Kendzor et al., 2009) suggest stress is one pathway through which neighborhood disadvantage may affect alcohol use and problems. Furthermore, differential vulnerability of some African Americans and other racial/ethnic minority group members to stress may result from compounding forms and prolonged exposure to disadvantage. A longitudinal study of young adults demonstrated effects of acute exposure to neighborhood poverty on heavy alcohol use accompanied by an additional effect of cumulative exposure to neighborhood poverty over 20 years (Cerda et al., 2010).
Second, disadvantaged neighborhoods suffer from proliferation of alcohol outlets including bars and liquor stores. Greater availability of alcohol is associated with increased alcohol use (Bryden et al., 2012) and a variety of alcohol-related problems (Theall et al., 2009). African Americans differentially may be at-risk for problems such as injury or liver problems in areas with higher densities of off-premise alcohol outlets (Theall et al., 2009). One study of northern California found bars with primarily African American patrons served larger spirits-based drinks than those with primarily White patrons or a more racially-diverse clientele (Kerr et al., 2008), perhaps further contributing to disparities in alcohol-related problems associated with increased alcohol outlet density for African Americans. Disadvantaged neighborhoods also evidence a lack of social control on deviant behaviors, which may promote permissive norms around heavy alcohol use and drunkenness, particularly in the context of easy access to alcohol.
In addition to overall alcohol outlet density, promotion of high-alcohol content beverages also may contribute to disparities in alcohol problems. Neighborhoods with a high density of African Americans, often some of the most socioeconomically disadvantaged, are targeted for marketing of high-alcohol content beverages, including malt liquor and distilled spirits (McKee et al., 2011). Drinking high-alcohol content beverages can lead to increased alcohol-related problems. In the U.S., African Americans are some of the largest consumers of malt liquor products (Bluthenthal et al., 2005). In disadvantaged neighborhoods, the combination of stress and permissive norms, with pervasive marketing of and easy access to alcohol, produces high-risk consumption patterns that are likely contributors to disparities in alcohol problems.
As most research on neighborhood effects on alcohol outcomes has been cross-sectional, it is important to note that effects may be partly due to social migration. That is, people who drink heavily also are more likely to move to disadvantaged areas over time (Buu et al., 2007). This downward social mobility (neighborhood selection effect) is likely to happen in addition to effects on alcohol problems caused by residence in disadvantaged neighborhoods (neighborhood causal effect).
DRINKING CULTURE AND CONTEXTS
Some geographic regions represent a microcosm, where unique configurations of individual and environmental risk and protective factors influence racial/ethnic vulnerability to alcohol problems in ways that are not seen elsewhere. The U.S.-Mexico border is one such area. In other U.S. regions, variations in drinking norms, religion, socioeconomic status, and alcohol control policies may have differential influences on residents’ drinking behaviors. There are more than seven million residents of the U.S.-Mexico border region at elevated risk for problematic alcohol use. Border residents are younger, undereducated, and more likely to live in poverty than residents of other areas of the U.S. The border population is also predominately Mexican American, a group that reports higher levels of alcohol use and alcohol problems than most other Hispanic national groups (Caetano and Mills, 2011). These characteristics represent known individual risk factors for alcohol use and alcohol-related problems. Although residence in high Hispanic-density neighborhoods is an environmental factor that reduces the risk for AUDs among Hispanics (Molina et al., 2012), a plethora of other environmental risk factors in the border region, spanning national alcohol policy to common local drinking contexts, appear to mitigate this protective effect.
For example, Mexico’s lower legal drinking age of 18 makes it attractive to younger U.S. residents who can consume relatively inexpensive alcohol there legally (Lange et al., 2002). Lax controls over public intoxication and rowdy behavior promote risky drinking behavior, and marketing tactics of bars specifically target younger patrons and their distinct patterns of intermittent, heavy drinking (Lange and Voas, 2000). Drinking in bars is associated with increased alcohol consumption and alcohol-related problems such as fights, driving under the influence (DUI), and sexual victimization of women (e.g., Gruenewald et al., 1999). Furthermore, alcohol-related aggression is more likely in bars frequented by younger people and by groups of males, two common characteristics of border residents who cross the border to drink. Aggressive behavior also is more likely with crowding or permissive behavioral expectations regarding intoxication (for a review of these findings, see Graham et al., 2006), both of which are common contextual characteristics of border drinking locations.
Expectations of elevated risk among younger and Mexican American border residents have been empirically confirmed. For example, on the California-Mexico border, roughly half of all 18–20 year olds crossed the border to drink in Tijuana bars in the past year, and Hispanics were significantly more likely to do so (64%) than other ethnic groups (Lange et al., 2002). Similarly, over 25% of Hispanic youth returning from an evening on the Mexico side of the border had a BAC exceeding .08 (Lange and Voas, 2000). In comparing representative samples of Mexican Americans along the border and in other areas of the U.S., border residence predicts increased alcohol use and problems (Wallisch and Spence, 2006), and recent findings suggest the effects are strongest among young adults (Caetano et al., 2012, Caetano et al., in press).
The findings reviewed above demonstrate elevated risk for alcohol problems on the border and suggest that a specific environmental context – drinking at bars – may play a particularly important role. Recent studies are providing insights into how this factor may be contributing to this disparity. First, it does not appear to reflect direct attempts to take advantage of Mexico’s lower legal drinking age or cheaper alcohol, as the number of current drinking Mexican Americans who cross into Mexico to drink alcohol (36% annually) does not fully account for the elevated risk observed in this population (Caetano et al., 2013). Moreover, it does not appear to reflect regional variation in known cultural or social-cognitive antecedent correlates of drinking behavior. For example, the disparity remains after adjusting for regional differences in religious preference (Caetano et al., 2012) and alcohol-related attitudes, beliefs, norms, and motives. These factors, which are important to acculturation-related increases in drinking among Hispanics generally (Zemore, 2007), are largely comparable between Mexican Americans in border and non-border locations. In contrast, patterns of bar attendance appear to uniquely explain the disparity (Mills et al., 2012).
By creating opportunities for younger border residents to drink that are not typically present in other geographic areas, bars on either side of the border appear to reflect a strong environmental influence on regional alcohol use. Studies to map the distribution of alcohol outlets on the border will help to clarify these effects.
CONCLUSIONS
This review presents some of the factors associated with racial/ethnic group drinking behaviors and that influence racial/ethnic group differences in drinking and alcohol-related problems. Specific illustrations in this review consider research in Asian Americans and in Hispanics living on and off the border, and research across African Americans and Whites related to stress and drinking, as well as across Hispanics in relation to neighborhood effects. It responds to the National Institute on Alcohol Abuse and Alcoholism’s (2010) Strategic Plan to Address Health Disparities, which calls for research aimed at reducing health disparities for alcohol in racial/ethnic minority groups. A more comprehensive review could detail other factors associated with racial/ethnic drinking behaviors and consequences, including levels of acculturation and motives for drinking (e.g., to cope), and other racial/ethnic groups (e.g., Native Americans) or the heterogeneity within and between groups in countries of origin and in drinking customs and norms.
Factors associated with alcohol use and problems require a multi-level perspective; those reviewed here and presented in Figure 1 ranged from biological pathways (e.g., alcohol metabolism and the serotonin system) to individual factors like age and antisocial behavior, the social context (e.g., drinking norms and exposure to stressors), and physical context in relation to access to alcohol and drinking in bars. The relationships between these factors in predicting drinking in racial/ethnic groups are complex. For example, biological factors (e.g., ALDH2*2) act together with individual and social factors to affect AUDs in Asians. Several mechanisms including increased stress and the marketing of high alcohol-content beverages are hypothesized to link neighborhood disadvantage to drinking and alcohol consequences, which may be associated with greater consequences for African Americans. An accumulation of multiple risk factors in the U.S.-Mexico border region may compound vulnerability for problematic alcohol use.
We suggest that this level of complexity requires an interdisciplinary research approach. The researchers involved in this review are from biological, clinical, behavioral, psychosocial, and population science backgrounds. Models for these types of collaborations, including in health disparities and cancer research, exist within the National Institutes of Health (Warnecke et al., 2008). Building new collaborations and interdisciplinary teams is a potential next step for moving this area of research forward. Interdisciplinary research is relevant to the study of gene by environment interactions, as the associations of biological mechanisms with individual, family, community, and cultural factors are often considered. Studies of gene x neighborhood effects on drinking could be one example. Neighborhood residence is often cited as an explanation for racial/ethnic group differences in health, and twin studies report larger effects for genetic factors on alcohol consumption with an urban versus rural residency and in communities with younger residents and greater migration (e.g., Dick et al., 2001).
The ultimate goal for alcohol health disparities research is to eliminate the disproportionate consequences from drinking experienced in some racial/ethnic minority groups. These findings may offer preliminary implications for policies or prevention and intervention programs. To our knowledge, however, this is a first effort at bringing together research on biological, individual, and social factors, including the macro and micro environment, associated with alcohol outcomes in U.S. racial/ethnic groups. This review offers a starting point, but additional research including prospective studies is clearly needed. Epigenetics is one area for increased focus in health disparities research. Epigenetics involves changes in chromatin structure (associated with environmental cues, e.g., in utero alcohol exposure, chronic alcohol use, and high blood alcohol levels) that affect gene expression via DNA methylation and/or histone modifications without changing the underlying DNA sequence (Kobor and Weinberg, 2011). The relationship of epigenetic mechanisms to fetal alcohol spectrum disorder and alcoholic liver disease – two alcohol-related diseases observed at higher rates in Blacks and Hispanics, respectively, compared with Whites – are being considered (for review see Kobor and Weinberg, 2011, Mandrekar, 2011).
Furthermore, the investigation of gene by stress interaction and alcohol consumption could have implications for the development of both personalized and community-level prevention and intervention programs to reduce AUDs (Brody et al., 2009), thereby translating gene-environment interaction knowledge into practice. A better understanding of individual and social risk and protective factors provides important information about who is most vulnerable to adverse alcohol consequences and helps improve prevention and intervention efforts targeted to a specific racial/ethnic group (e.g., African Americans) or a subgroup within an ethnicity (e.g., Korean Americans and Mexican Americans). Risk and protective factors for alcohol-related problems include the macro-environment and physical context. Modifiable risk factors at the macro-level include bar attendance for younger drinkers and the spatial density of alcohol outlets in a geographic region. Preventive interventions can also be targeted to racial/ethnic minority group members residing in disadvantaged areas, and economic development policy and alcohol control efforts may need to be more closely linked to help eliminate disparities in alcohol problems. While these strategies are feasible, they do not fully account for the complex interrelationships between factors at different levels, from biological to physical context. More research is needed to prioritize combinations of multi-level targets with the most promise to address health disparities in racial/ethnic minority groups.
Acknowledgments
This work was supported by National Institutes of Health grants K01AA021145 to KGC, R21AA017584 to DMS and JC, K02AA00269 and R01AA11257l to TLW, R21AA019175 to KJK, and R01AA013642 to RC
Footnotes
The subject of this mini-review was presented at the scientific meeting of the Research Society on Alcoholism, June 23–27, 2012 (San Francisco, CA). Organizers and chairs of the symposium were Karen Chartier and Denise Scott. Introducer was Karen Chartier. Speakers were Jonathan Covault, Tamara Wall, Britain Mills, and Katherine Karriker-Jaffe. Discussant was Judith Arroyo.
References
- BINDER EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- BLUTHENTHAL RN, BROWNTAYLOR D, GUZMÁM-BECERRA N, ROBINSON PL. Characteristics of malt liquor beer drinkers in a low-income, racial minority community sample. Alcoholism: Clinical and Experimental Research. 2005;29:402–409. doi: 10.1097/01.alc.0000156118.74728.34. [DOI] [PubMed] [Google Scholar]
- BRODY GH, BEACH SR, PHILIBERT RA, CHEN YF, MURRY VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child Dev. 2009;80:645–61. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- BROWN RL, RICHMAN JA. Sex differences in mediating and moderating processes linking economic stressors, psychological distress, and drinking. Journal of Studies on Alcohol and Drugs. 2012;73:811–819. doi: 10.15288/jsad.2012.73.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYDEN A, ROBERTS B, MCKEE M, PETTICREW M. A systematic review of the influence on alcohol use of community level availablity and marketing of alcohol. Health & Place. 2012;18:349–357. doi: 10.1016/j.healthplace.2011.11.003. [DOI] [PubMed] [Google Scholar]
- BUU A, MANSOUR M, WANG J, REFIOR SK, FITZGERALD HE, ZUCKER RA. Alcoholism effects on social migration and neighborhood effects on alcoholism over the course of 12 years. Alcoholism: Clinical and Experimental Research. 2007;31:1545–1551. doi: 10.1111/j.1530-0277.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAETANO R, MILLS BA. The Hispanic Americans Baseline Alcohol Survey (HABLAS): Is the “prevention paradox” applicable to alcohol problems across Hispanic national groups? Alcoholism: Clinical and Experimental Research. 2011;35:1256–64. doi: 10.1111/j.1530-0277.2011.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAETANO R, MILLS BA, VAETH PAC. Alcohol consumption and binge drinking among U.S.-Mexico border and non-border Mexican Americans. Alcoholism: Clinical and Experimental Research. 2012;36:677–685. doi: 10.1111/j.1530-0277.2011.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAETANO R, MILLS BA, VAETH PAC. Alcohol use among Mexican American U.S.-Mexico border residents: Differences between those who drink and who do not drink in Mexico. Addictive Behaviors. 2013;38:2026–2031. doi: 10.1016/j.addbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAETANO R, VAETH PAC, MILLS BA, RODRIGUEZ LA. Alcohol abuse and dependence among U.S.-Mexico border and non-border Mexican Americans. Alcoholism: Clinical and Experimental Research. doi: 10.1111/acer.12061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPI A, HARIRI AR, HOLMES A, UHER R, MOFFITT TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERDA M, DIEZ-ROUX AV, TCHETGEN ET, GORDON-LARSEN P, KIEFE C. The relationship between neighborhood poverty and alcohol use: estimation by marginal structural models. Epidemiology. 2010;21:482–9. doi: 10.1097/EDE.0b013e3181e13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARTIER K, CAETANO P. Ethnicity and health disparities in alcohol research. Alcohol Res Health. 2010;33:152–60. [PMC free article] [PubMed] [Google Scholar]
- COCHRANE J, CHEN H, CONIGRAVE KM, HAO W. Alcohol use in China. Alcohol Alcohol. 2003;38:537–42. doi: 10.1093/alcalc/agg111. [DOI] [PubMed] [Google Scholar]
- COOPER ML, KRULL JL, AGOCHA VB, FLANAGAN ME, ORCUTT HK, GRABE S, DERMEN KH, JACKSON M. Motivational pathways to alcohol use and abuse among Black and White adolescents. J Abnorm Psychol. 2008;117:485–501. doi: 10.1037/a0012592. [DOI] [PubMed] [Google Scholar]
- COVAULT J, TENNEN H, ARMELI S, CONNER TS, HERMAN AI, CILLESSEN AH, KRANZLER HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–16. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- DICK DM, ROSE RJ, VIKEN RJ, KAPRIO J, KOSKENVUO M. Exploring gene-environment interactions: socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:625–32. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- ENG MY, LUCZAK SE, WALL TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–7. [PMC free article] [PubMed] [Google Scholar]
- GRAHAM K, BERNARDS S, OSGOOD DW, WELLS S. Bad nights or bad bars? Multi-level analysis of environmental predictors of aggersstion in late-night large-capacity bars and clubs. Addiction. 2006;101:1569–1580. doi: 10.1111/j.1360-0443.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- GRUENEWALD PJ, STOCKWELL T, BEEL A, DYSKIN EV. Beverage sales and drinking and driving: the role of on-premise drinking places. Journal of Studies on Alcohol. 1999;60:47–53. doi: 10.15288/jsa.1999.60.47. [DOI] [PubMed] [Google Scholar]
- HAHM BJ, CHO MJ. Prevalence of alcohol use disorder in a South Korean community--changes in the pattern of prevalence over the past 15 years. Soc Psychiatry Psychiatr Epidemiol. 2005;40:114–9. doi: 10.1007/s00127-005-0854-9. [DOI] [PubMed] [Google Scholar]
- HASIN DS, STINSON FS, OGBURN E, GRANT BF. Prevalence, correlates, disability and comorbidity of DSM IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- HELZER JE, CANINO GJ, YEH EK, BLAND RC, LEE CK, HWU HG, NEWMAN S. Alcoholism- North America and Asia. Archives of General Psychiatry. 1990;47:313–319. doi: 10.1001/archpsyc.1990.01810160013002. [DOI] [PubMed] [Google Scholar]
- HIGUCHI S, MATSUSHITA S, IMAZEKI H, KINOSHITA T, TAKAGI S, KONO H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–2. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- HURLEY TD, EDENBERG HJ. Genes encoding enzymes involved in ethanol metabolism. Alcohol Res. 2012;34:339–44. [PMC free article] [PubMed] [Google Scholar]
- HWU HG, YEH EK, CHANG LY. Prevalence of psychiatric disorders in Taiwan defined by the Chinese Diagnostic Interview Schedule. Acta Psychiatr Scand. 1989;79:136–47. doi: 10.1111/j.1600-0447.1989.tb08581.x. [DOI] [PubMed] [Google Scholar]
- JONES-WEBB R, SNOWDEN LR, HERD D, SHORT B, HANNAN P. Alcohol-related problems among black, Hispanic, and white men: the contribution of neighborhood poverty. Journal of Studies on Alcohol. 1997;58:539–545. doi: 10.15288/jsa.1997.58.539. [DOI] [PubMed] [Google Scholar]
- KARRIKER-JAFFE KJ. Areas of disadvantage: a systematic review of effects of area-level socioeconomic status on substance use. Drug and Alcohol Review. 2011;30:84–95. doi: 10.1111/j.1465-3362.2010.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARRIKER-JAFFE KJ, ZEMORE SE, MULIA N, JONES-WEBB R, BOND J, GREENFIELD TK. Neighborhood disadvantage and adult alcohol outcomes: differential risk by race and gender. J Stud Alcohol Drugs. 2012;73:865–73. doi: 10.15288/jsad.2012.73.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDZOR DE, BUSINELLE MS, MAZAS CA, COFTA-WOERPEL LM, REITZEL LR, VIDRINE JI, LI Y, COSTELLO TJ, CINCIRIPINI PM, AHLUWALIA JS, WETTER DW. Pathways between socioeconomic status and modifiable risk factors among African American smokers. Journal of Behavioral Medicine. 2009;32:545–557. doi: 10.1007/s10865-009-9226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR WC, PATTERSON D, KOENEN MA, GREENFIELD TK. Alcohol content variation of bar and restaurant drinks in Northern California. Alcoholism: Clinical and Experimental Research. 2008;32:1623–1629. doi: 10.1111/j.1530-0277.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBOR MS, WEINBERG J. Focus on: epigenetics and fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34:29–37. [PMC free article] [PubMed] [Google Scholar]
- KRANZLER HR, FEINN R, NELSON EC, COVAULT J, ANTON RF, FARRER L, GELERNTER J. A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:960–8. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANZLER HR, SCOTT D, TENNEN H, FEINN R, WILLIAMS C, ARMELI S, TAYLOR RE, BRIGGS-GOWAN MJ, COVAULT J. The 5-HTTLPR polymorphism moderates the effect of stressful life events on drinking behavior in college students of African descent. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:484–90. doi: 10.1002/ajmg.b.32051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGE JE, VOAS RB. Youth escaping limits on drinking: Binging in Mexico. Addiction. 2000;95:521–8. doi: 10.1046/j.1360-0443.2000.9545214.x. [DOI] [PubMed] [Google Scholar]
- LANGE JE, VOAS RB, JOHNSON MB. South of the border: A legal haven for underage drinking. Addiction. 2002;97:1195–203. doi: 10.1046/j.1360-0443.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- LEE CK, KWAK YS, YAMAMOTO J, RHEE H, KIM YS, HAN JH, CHOI JO, LEE YH. Psychiatric epidemiology in Korea. Part I: Gender and age differences in Seoul. J Nerv Ment Dis. 1990;178:242–6. doi: 10.1097/00005053-199004000-00004. [DOI] [PubMed] [Google Scholar]
- LINDELL SG, SCHWANDT ML, SUN H, SPARENBORG JD, BJORK K, KASCKOW JW, SOMMER WH, GOLDMAN D, HIGLEY JD, SUOMI SJ, HEILIG M, BARR CS. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2010;67:423–31. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCZAK SE, GLATT SJ, WALL TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;132:607–21. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- LUCZAK SE, WALL TL, COOK TAR, SHEA SH, CARR LG. ALDH2 status and conduct disorder mediate the relationship between ethnicity and alcohol dependence in Chinese-, Korean-, and White-American college students. Journal of Abnormal Psychology. 2004;113:271–278. doi: 10.1037/0021-843X.113.2.271. [DOI] [PubMed] [Google Scholar]
- MANDREKAR P. Epigenetic regulation in alcoholic liver disease. World J Gastroenterol. 2011;17:2456–64. doi: 10.3748/wjg.v17.i20.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKEE P, JONES-WEBB R, HANNAN P, PHAM L. Malt liquor marketing in inner cities: The role of neighborhood racial composition. Journal of Ethnicity in Substance Abuse. 2011;10:24–38. doi: 10.1080/15332640.2011.547793. [DOI] [PubMed] [Google Scholar]
- MILLS BA, CAETANO R, VAETH PAC. What explains higher levels of drinking among Mexican Americans on the U.S.-Mexico border? Alcoholism: Clinical and Experimental Research. 2012;36:S258. [Google Scholar]
- MOLINA KM, ALEGRÍA M, CHEN CN. Neighborhood context and substance use disorders: A comparative analysis of racial and ethnic groups in the United States. Drug and Alcohol Dependence. 2012;125S:S35–S43. doi: 10.1016/j.drugalcdep.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATIONAL INSTITUTE ON ALCOHOL ABUSE AND ALCOHOLISM [NIAAA] NIAAA’s Strategic Plan to Address Health Disparities. Bethesda, MD: NIAAA; 2010. [Google Scholar]
- ROBERTS AL, GILMAN SE, BRESLAU J, BRESLAU N, KOENEN KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41:71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARI Y, JOHNSON VR, WEEDMAN JM. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog Mol Biol Transl Sci. 2011;98:401–43. doi: 10.1016/B978-0-12-385506-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, FOX HC, HONG KI, HANSEN J, TUIT K, KREEK MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEALL KP, SCRIBNER R, COHEN D, BLUTHENTHAL RN, SCHONLAU M, LYNCH S, FARLEY TA. The neighborhood alcohol environment and alcohol-related morbidity (doi: 10.1093/alcalc/agp042) Alcohol and Alcoholism. 2009;44:491–499. doi: 10.1093/alcalc/agp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL TL. Genetic associations of alcohol and aldehyde dehydrogenase with alcohol dependence and their mechanisms of action. Ther Drug Monit. 2005;27:700–3. doi: 10.1097/01.ftd.0000179840.78762.33. [DOI] [PubMed] [Google Scholar]
- WALL TL, CARR LG, EHLERS CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am J Psychiatry. 2003;160:41–6. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- WALLISCH LS, SPENCE RT. Alcohol and drug use, abuse and dependence in urban areas and colonias of Texas-Mexico border. Hispanic Journal of Behavioral Sciences. 2006;28:286–307. [Google Scholar]
- WARNECKE RB, OH A, BREEN N, GEHLERT S, PASKETT E, TUCKER KL, LURIE N, REBBECK T, GOODWIN J, FLACK J, SRINIVASAN S, KERNER J, HEURTIN-ROBERTS S, ABELES R, TYSON FL, PATMIOS G, HIATT RA. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE P, KRANZLER HR, POLING J, STEIN MB, ANTON RF, FARRER LA, GELERNTER J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–92. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEMORE SE. Acculturation and alcohol among Latino adults in the United States: a comprehensive review. Alcohol Clin Exp Res. 2007;31:1968–90. doi: 10.1111/j.1530-0277.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- ZHOU Z, ZHU G, HARIRI AR, ENOCH MA, SCOTT D, SINHA R, VIRKKUNEN M, MASH DC, LIPSKY RH, HU XZ, HODGKINSON CA, XU K, BUZAS B, YUAN Q, SHEN PH, FERRELL RE, MANUCK SB, BROWN SM, HAUGER RL, STOHLER CS, ZUBIETA JK, GOLDMAN D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]