Congenital facial weakness in Moebius syndrome is accompanied by ocular abduction deficits attributed to abducens nerve agenesis. Rucker et al. present a new classification scheme, revealing that the most common deficit is bilateral horizontal gaze palsy from abducens nuclear defects and identifying vertical abnormalities suggestive of midbrain involvement.
Keywords: Moebius syndrome, sixth nerve palsy, horizontal gaze palsy, congenital cranial dysinnervation disorders, congenital facial weakness, saccades
Abstract
Congenital facial weakness is present in a heterogeneous group of conditions. Among them is Moebius syndrome, which has been defined as a disorder with congenital, non-progressive facial weakness and limited abduction of one or both eyes. It is typically attributed to agenesis of the abducens and facial cranial nerves. This paper details ocular motor findings of 40 subjects (23 months to 64 years; 24 females, 16 males) with congenital facial weakness: 38 presented at a Moebius Syndrome Conference and two were clinic patients. A new classification scheme of patterns based on ocular motor phenotype is presented. Of 40 subjects, 37 had bilateral and three had unilateral facial weakness. The most common ocular motor pattern (Pattern 1, n = 17, 43%) was bilateral horizontal gaze palsy with intact vertical range. Pattern 2 (n = 10, 26%) was bilateral horizontal gaze palsy with variable vertical limitations. Pattern 3, which was rare, was isolated abduction deficits (n = 2, 5%). Others had full motility range and did not meet minimal criteria for the diagnosis of Moebius syndrome (Pattern 4, n = 10, 26%). One subject was too severely affected to characterize. Abnormal vertical smooth pursuit was present in 17 (57%) of 30 subjects: nine with Pattern 1, five with Pattern 2, and three with Pattern 4. Abnormal vertical saccades were present in 10 (34%) of 29 subjects. Vertical saccades appeared slow in nine: six with Pattern 1 and three with Pattern 2. Vertical saccades were absent in one subject with Pattern 2. Abnormal vertical optokinetic nystagmus was present in 19 (68%) of 28 subjects: 10 with Pattern 1, six with Pattern 2, one with Pattern 3, and two with Pattern 4. Reduced convergence was present in 19 (66%) of 29 subjects: nine with Pattern 1, six with Pattern 2, one with Pattern 3, and three with Pattern 4. The most common pattern of ocular motor deficit in Moebius syndrome is bilateral horizontal gaze palsy from pontine abducens nuclear defects, rather than abducens nerve involvement. Defects in the range or dynamic properties of vertical movements in subjects with congenital facial weakness may suggest involvement of ocular motor structures in the midbrain, including oculomotor nerves or nuclei, vertical supranuclear saccadic centres, and convergence neurons. Such deficits were found even in subjects with full vertical motility range. Classification of patterns of ocular motor deficits in congenital facial weakness may assist with further delineation of anatomic localization and identification of genetic deficits underlying these disorders.
Introduction
Congenital facial weakness is uncommon and can be a feature of a diverse group of disorders. One such condition is Moebius syndrome (also referred to in the literature as Moebius sequence; Stromland et al., 2002; Miller et al., 2005; Ventura et al., 2012), originally described by Alfred Graefe (1880) but eponymously named after the German neurologist Paul Julius Möbius (Möbius, 1888). It has been defined as a developmental disorder with obligate congenital, non-progressive facial weakness and limited abduction of one or both eyes (Miller, 2007). These features are typically attributed to agenesis of the abducens (sixth) and facial (seventh) cranial nerves; however, pontine abducens nuclear involvement with resultant horizontal gaze palsy may be more common (Verzijl et al., 2003; de Souza-Dias and Goldchmit, 2007). In addition, dynamic eye movements such as saccades, optokinetic nystagmus and convergence that may clarify phenotypes and help localize affected ocular motor structures are not well characterized. Moebius syndrome falls within a broader spectrum of congenital cranial dysinnervation disorders, which have received significant attention in recent years as a result of the discovery of causative genetic mutations (Gutowski et al., 2003; Yamada et al., 2003, 2004; Tischfield et al., 2005; Demer et al., 2007; Tischfield et al, 2010; Webb et al., 2012; Chew et al., 2013) and in which ocular motor phenotyping is playing a prominent role in targeting genetic screening. The objectives of the current study were to systematically characterize ocular motor findings in a large group of subjects with congenital facial weakness and to establish categorical patterns of eye movement abnormalities, with the goal of using such phenotypic information to perform targeted genetic studies in the future.
Materials and methods
This is a cross-sectional observational study of 38 subjects with congenital facial weakness present at the 10th Moebius Syndrome Conference (2012). The conference was sponsored by the Moebius Syndrome Foundation. Two additional subjects were evaluated at Mount Sinai Medical Centre after the conference. Informed consent was obtained from all subjects according to the Declaration of Helsinki and the study was approved by the Icahn School of Medicine Institutional Review Board.
Forty subjects with congenital facial weakness underwent systematic historical neurological questioning and neuro-ophthalmologic examination by a paediatric ophthalmologist (T.F.) and a neuro-ophthalmologist (J.C.R.). Near visual acuity, colour vision (Ishihara PseudoIsochromatic Plates), pupillary size and reactivity, eyelid position and palpebral fissure width in three gaze positions (central, right and left) were assessed when possible (young subject age precluded detailed testing in some subjects). Near visual acuity results are reported as distance equivalents. Ocular motor examination consisted of clinical examination and photographic assessment of the range of eye movements in five gaze positions (centre, up, down, right, left) and ocular alignment (cover and cross-cover testing with prism measurements or Krimsky method if the subject was too young to cooperate with cover testing). Based on ocular motor range testing, subjects were classified into four Patterns: Pattern 1 with BHGP (bilateral horizontal gaze palsy) and full vertical range; Pattern 2 with BHGP and vertical range limitations; Pattern 3 with limitation of abduction range only; and Pattern 4 with full ocular motility range. Dynamic assessment of smooth pursuit, saccades, optokinetic nystagmus, and convergence was performed by clinical examination and by review of high-speed infrared video recordings. Handheld small red targets were used for smooth pursuit, saccade and convergence testing and a red/white striped cloth was used for optokinetic nystagmus testing. Saccades were tested as subjects were asked to look between two stationary targets. Convergence was tested as subjects were asked to follow a target moving from distance to near. Both fast and slow phases of optokinetic nystagmus were evaluated, as was any tendency for the eyes to deviate (e.g. in the direction of stripe movement). Dynamic data capture was limited in small children. Neurological examination included facial sensation, tongue protrusion and anatomy, strength grading, sensory exam, reflexes, finger-to-nose testing, and casual and tandem gait. Subjects were also examined for mirror movements, as well as dysmorphic features of Poland anomaly (pectoralis hypoplasia and ipsilateral syndactyly; Parker et al., 1981; Bavinck and Weaver, 1986), and major limb malformations such as club foot, limb reduction defects, or complete syndactyly/fusion of digits.
Results
The 40 subjects included 24 (60%) females and 16 (40%) males ranging in age from 23 months to 64 years (mean 20.6 ± 17.2 years; Supplementary Table 1). Of 40 subjects, 37 (92.5%) had bilateral and three (7.5%) unilateral left-sided facial weakness. Five subjects, representing two families, had affected siblings who also participated in this study. All subjects had near visual acuity of 20/25 or better or were able to fix and follow, except for two subjects with acuity in the 20/30–20/40 range and one with dense amblyopia, with only light perception in the left eye. All subjects tested (n = 30) had normal colour vision, except for one male (4/14 Ishihara plates OU). All subjects had normal pupils. One subject had ptosis (bilateral, severe).
Ocular motor range, alignment and aberrant movement
Twenty-nine subjects (74%) met the minimum diagnostic criteria for Moebius syndrome: facial weakness and ocular abduction weakness. One subject was too severely affected cognitively for detailed ocular motor characterization by examination; however, ocular motor range appeared limited in all directions and the subject’s guardian substantiated lack of eye movements. For the remaining 39 subjects, the range of eye movement in horizontal and vertical directions was classified into four distinct patterns (Table 1). The most common ocular motor pattern (Pattern 1) was BHGP (bilateral horizontal gaze palsy) with intact vertical range of motion. This was present in 17 subjects (43%; Fig. 1). In subjects with Pattern 1, abduction and adduction were impaired to an equal degree in 10, whereas abduction was affected more severely than adduction in seven. Pattern 2, present in 10 subjects (26%) was BHGP with variable vertical limitations (Fig. 2 and Table 1). In subjects with Pattern 2, abduction and adduction were impaired to an equal degree in four, abduction more severely than adduction in three, and adduction more severely than abduction in three. All three subjects with adduction affected more severely than abduction had a severe limitation of elevation and depression. No subject had a deficit of vertical range in absence of BHGP. The single subject with bilateral ptosis had Pattern 2, with impaired elevation and depression of both eyes. This subject was previously noted to have the heterozygous E410 mutation in TUBB3 (Tischfield et al., 2010; Chew et al., 2013). Pattern 3, the defined Moebius syndrome with isolated abduction deficits, was rare (n = 2, 5%; Fig. 3). Pattern 4 consisted of full ocular motility range that does not meet the minimal criteria for the definition of Moebius syndrome. This was present in 10 subjects (26%). Subjects with Pattern 4 included three previously published subjects with accommodative esotropia and a homozygous R207C mutation in HOXB1, now defined as hereditary congenital facial paresis, type 3 (OMIM #614744; Webb et al., 2012). In the Pattern 4 group, there were two families, one with two affected siblings and one with three affected siblings. Ocular alignment was highly variable in Patterns 1, 2 and 4 (Table 1). Both subjects with Pattern 3 had normal ocular alignment in central position in the absence of a history of strabismus surgery.
Table 1.
Ocular motor patterns in congenital facial weakness
| Pattern | n (%) | Horizontal range | Vertical range | Alignment | Vertical smooth pursuit Number abnormal (%) [Number tested] | Vertical saccades Number abnormal (%) [Number tested] | Vertical OKN Number abnormal (%) [Number tested] | Convergence Number abnormal (%) [Number tested] | Convergence substitution for versions (Cross-fixation) n, (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 (43%) | Bilateral horizontal gaze palsy | Normal | No surgery: 4 ortho, 2 eso, 3 exo, 1 hyper Post-surgical: 4 ortho, 1 eso, 1 eso/hyper, 1 exo/hyper | 9 (75%) [12] | 6 (55%) [11] | 10 (91%) [11] | 9 (75%) [12] | 2 (12%) |
| Slow in 6/6 | Absent in 5/10 | ||||||||
| Slow phases only in 5/10 | |||||||||
| 2a | 10 (26%) | Bilateral horizontal gaze palsy | Impaired | No surgery: 2 ortho | 5 (63%) [8] | 4 (50%) [8] | 6 (86%) [7] | 6 (75%) [8] | 1 (5%) |
| n = 4c – elevation and depressiona | Post-surgical: 1 ortho, 3 exoa, 2 hyper, 1 eso/hyper, 1 exo/hyper | Slow in 3/4 | Absent in 5/6 | ||||||
| Absent in 1/4 | Slow phases only in 1/6 | ||||||||
| n = 6d – elevation only | |||||||||
| 3 | 2 (5) | Impaired abduction | Normal | No surgery: 2 ortho | 0 (0%) [2] | 0 (0%) [2] | 1 (50%) [2] | 1 (50%) [2] | 1 (50%) |
| 4b | 10 (26%) | Normal | Normal | No surgery: 5 orthob, 2 exo | 3 (38%) [8] | 0 (0%) [8] | 2 (25%) [8] | 3 (43%) [7] | 0 (0%) |
| Post-surgical: 1 esob, 1 ortho (eso-near)b, 1 hyper |
aIncludes one subject with a confirmed TUBB3 genetic mutation. This subject had a residual exotropia, status-post strabismus surgery.
bIncludes three subjects with confirmed HOXB1 genetic mutation. Two were status-post strabismus surgery, one with residual esotropia and the other with esotropia at near only. One was orthophoric in the absence of a history of strabismus surgery.
cElevation deficits were bilateral in all subjects, depression deficits were bilateral in all but one subject.
dElevation deficits were unilateral in three subjects and bilateral in three subjects.
OKN = optokinetic nystagmus; ortho = orthophoric/no ocular misalignment; eso = inward-deviated ocular misalignment; exo = outward-deviated ocular misalignment; hyper = vertical ocular misalignment.
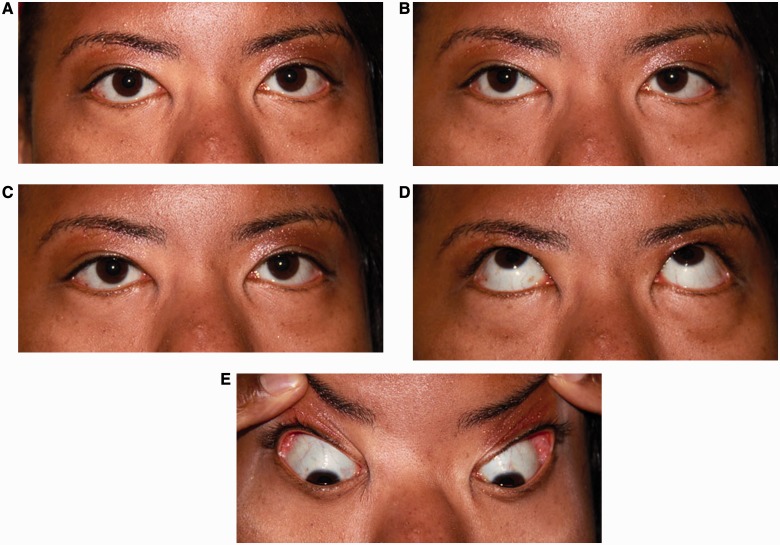
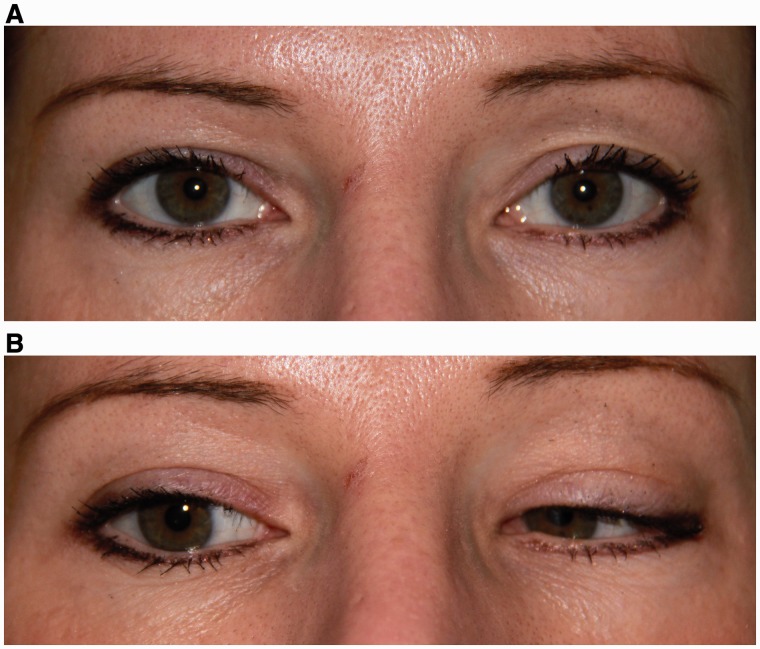
Figure 1.
Pattern 1. Bilateral horizontal gaze palsy. (A) Central position with normal ocular alignment. (B) Attempted right gaze with partial right horizontal gaze palsy, worse for abduction of the right eye than adduction of the left eye. (C) Attempted left gaze with complete left horizontal gaze palsy. (D) Normal elevation. (E) Normal depression.
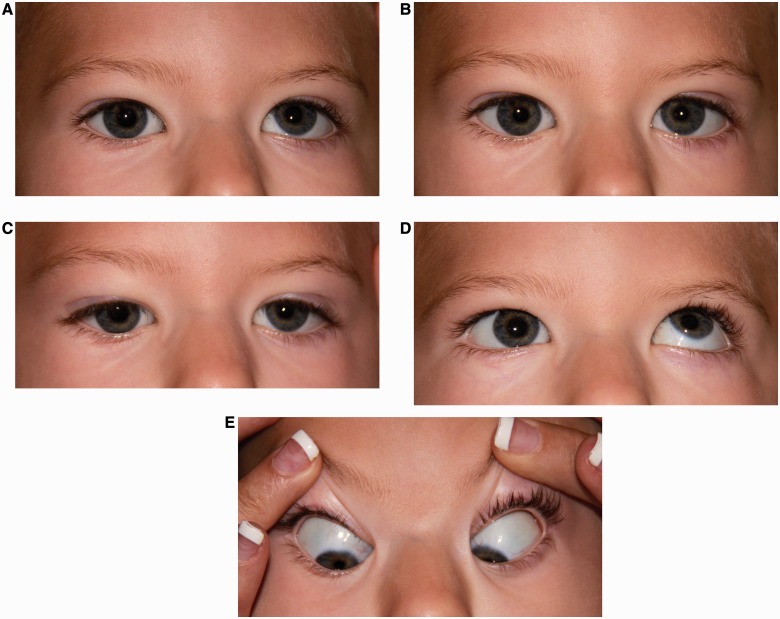
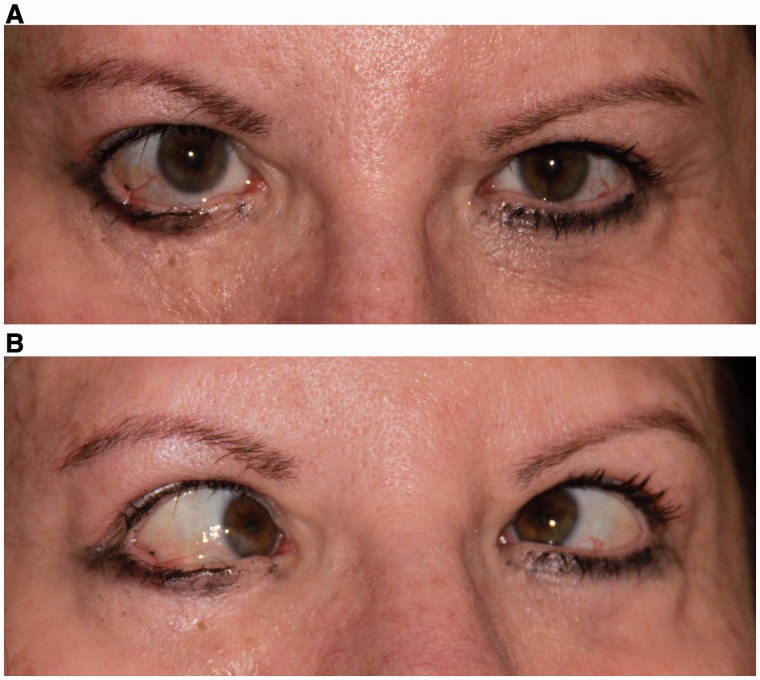
Figure 2.
Pattern 2. Bilateral horizontal gaze palsy with impaired vertical motility. (A) Central position with small left hypertropia. (B) Attempted right gaze with right horizontal gaze palsy, worse for abduction of the right eye than adduction of the left eye. (C) Attempted left gaze with left horizontal gaze palsy, worse for abduction of the left eye than adduction of the right eye. (D) Impaired elevation of the right eye with intact elevation of the left eye. (E) Normal depression.
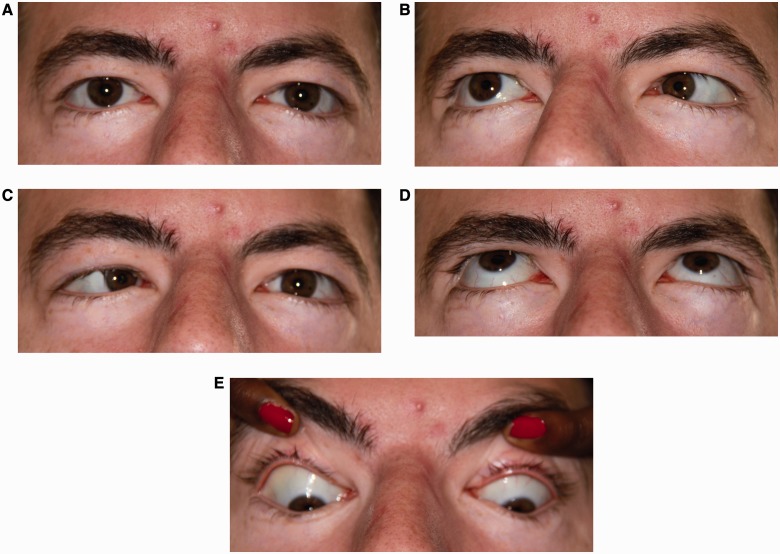
Figure 3.
Pattern 3. Impaired abduction. (A) Central position with normal ocular alignment. (B) Right gaze with impaired abduction of the right eye. (C) Left gaze with impaired abduction of the left eye. (D) Normal elevation. (E) Normal depression.
In addition to impaired range of motility, ocular motor pattern groups 1, 2 and 3 displayed additional types of aberrant motility upon attempted horizontal eye movement. These included abnormal elevation of one or both eyes (n = 5, 13%; Fig. 4), palpebral fissure narrowing (n = 5, 13%; Fig. 5), excessive convergence (n = 4, 10%; Fig. 6; Table 1), and globe retraction (n = 3, 8%). Prominent gaze-holding deficits and nystagmus were not observed.
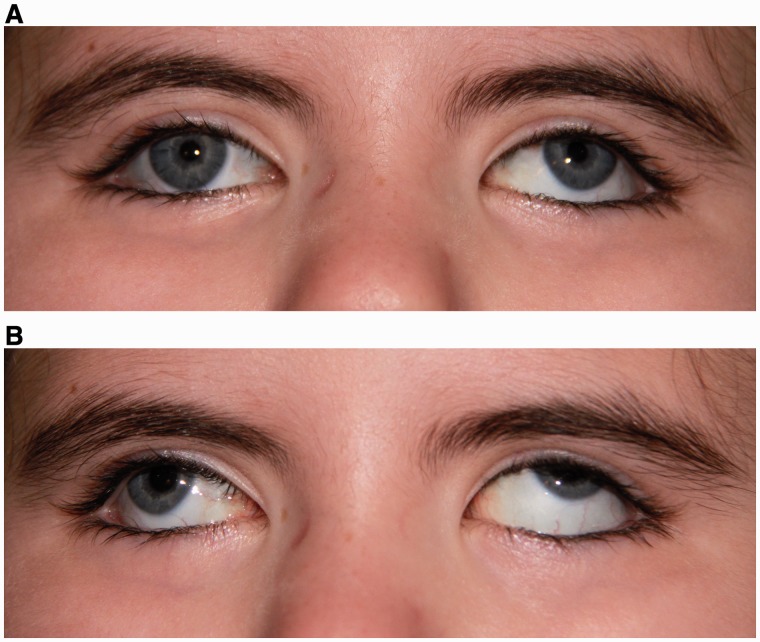
Figure 4.
Aberrant vertical motion with attempted horizontal gaze. (A) Central position with a large exotropia and left hypertropia. (B) Right horizontal gaze palsy with mild elevation of the right eye and marked elevation of the left eye upon attempted right gaze.
Figure 5.
Aberrant palpebral fissure narrowing with attempted horizontal gaze. (A) Baseline eye and lid position in central position. (B) Mild narrowing of the right palpebral fissure and marked narrowing of the left palpebral fissure on right gaze.
Figure 6.
Aberrant excessive convergence with attempted horizontal gaze. (A) Baseline eye position in central position. (B) Excessive convergence upon attempted left gaze.
Dynamic assessment of smooth pursuit, saccades, optokinetic nystagmus and convergence
Smooth pursuit was assessed in 30 subjects. Horizontal smooth pursuit was absent or abnormal in most subjects with BHGP and was normally generated in subjects with Pattern 4, with one exception. This subject (with hereditary congenital facial paresis, type 3) had impaired smooth pursuit with saccadic breakdown in both horizontal directions. Vertical smooth pursuit was abnormal in 17 subjects (57%), including subjects with Patterns 1, 2 and 4 (Table 1 and Supplementary Video 1). In three subjects with Pattern 1, smooth pursuit was impaired in the downward more than upward direction; in one Pattern 1 subject smooth pursuit was impaired in the upward more than downward direction; and in one subject with Pattern 4, smooth pursuit was impaired predominantly in the downward direction.
Saccades were assessed in 29 subjects. In all subjects with BHGP, horizontal saccades were either absent or slowed within the range of residual movement. Horizontal saccades appeared normal in all subjects with Pattern 4. One subject with Pattern 3 had slowed horizontal saccades in both directions. The second subject with Pattern 3 generated no horizontal saccades because of extreme convergence upon horizontal gaze attempts (Fig. 6). Vertical saccades were abnormal in 10 subjects (34%; Table 1 and Supplementary Video 1). Saccadic deficits seemed symmetric for upward and downward directions, with exception of one Pattern 2 subject, in whom only downward saccades were slowed.
Optokinetic nystagmus was assessed in 28 subjects. In all but two subjects with BHGP, horizontal optokinetic nystagmus was absent or substantially reduced. The two exceptions generated normal horizontal optokinetic nystagmus within the range of horizontal eye movement that remained. In one subject with BHGP, the horizontally-presented optokinetic nystagmus stimulus elicited convergent-retraction ocular motion. Vertical optokinetic nystagmus was abnormal in 19 subjects (68%; Table 1). Abnormal optokinetic nystagmus was either absent vertically, suggesting inability to follow the stripes, or a slow phase with eye motion only in the direction of the stripes was generated, suggesting inability to generate optokinetic nystagmus quick phases (Table 1). In three subjects (one with Pattern 1 and two with Pattern 2), downward optokinetic nystagmus was predominantly affected, with generation of a downward slow phase in the direction of stripe motion. The majority of subjects with absent optokinetic nystagmus demonstrated abnormal vertical smooth pursuit. Convergence was assessed in 29 subjects. Convergence capacity was reduced in 19 (66%; Table 1).
Additional neurological and systemic deficits
On a researcher-administered questionnaire and review of available medical records, imbalance was reported in 18 subjects (45%), hearing loss in nine (23%), moderate to severe mental retardation and intellectual disability in 10 (25%), mild cognitive delay and learning disabilities in eight (20%), and eye tearing with chewing in four (10%). Five of the nine with hearing loss had ocular motor Pattern 4. Of the nine subjects with moderate to severe mental retardation and intellectual disability who were able to cooperate with ocular motility range testing, two had Pattern 1, five had Pattern 2, and two had Pattern 4. Also reported were history of hypotonia (n = 3, 8%), mirror movements of the limbs (n = 3, 8%), psychiatric abnormalities (n = 3, 8%) including paranoia and repetitive behaviours, seizure disorder (n = 2, 5%), autism (n = 1, 3%), and vocal cord paralysis (n = 1, 3%). One subject required a wheelchair and one required a walker. In subjects with Pattern 4, two reported moderate to severe mental retardation and intellectual disability, two cognitive delay, one imbalance, and one repetitive behaviours. The remaining deficits were reported by subjects with abnormal ocular motor range.
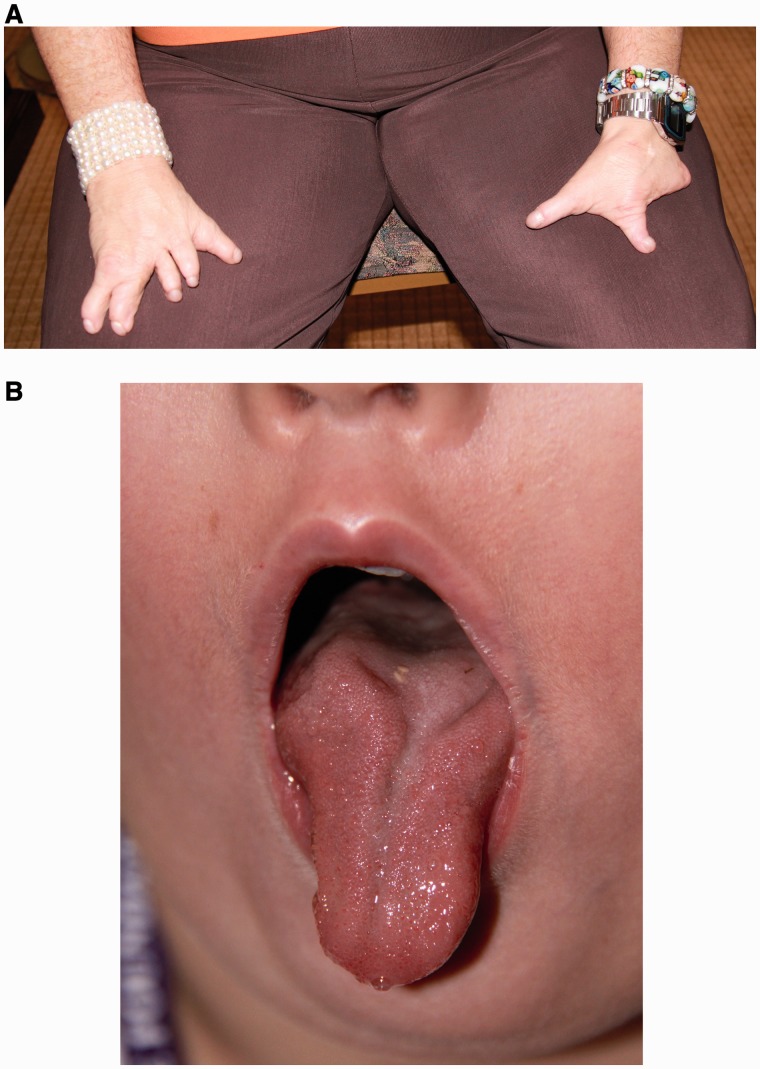
Full neurological examination was performed on 29 subjects. The remaining subjects were too young to fully cooperate. The most common abnormality was impaired tandem gait (n = 10, 34%), found in seven subjects with ocular motor Pattern 1, two with Pattern 2, and one with Pattern 3. Additional neurological findings, present only in patients with ocular motor Patterns 1 and 2 included: weakness (n = 6, 21%; proximal in three, distal in two, and a single limb in one), abnormal reflexes (n = 4, 14%; reduced in two, brisk in two), and unilateral trigeminal sensory deficits (n = 4, 14%). Severe limb anomalies such as club foot, limb reduction defects, or complete syndactyly were present in 18 subjects (45%; eight with Pattern 1, eight with Pattern 2, one with Pattern 3, and one with an uncharacterized ocular motor pattern) (Fig. 7A) and Poland anomaly was present in three subjects (8%; two with Pattern 1, one with Pattern 2), all of whom had abnormal ocular motility. The tongue was examined in 36 subjects, 21 (58%) of whom had abnormalities: 10 with ocular motor Pattern 1, eight with Pattern 2, both subjects with Pattern 3, and one with Pattern 4. The main abnormality was tongue atrophy, unilateral or bilateral, in 14 subjects (39%; Fig. 7B). Seven subjects (18%) had tongue deviation with protrusion. Three subjects (8%) had limited protrusion and one subject, the subject excluded from eye movement analysis because of severe mental retardation, had complete absence of the tongue.
Figure 7.
(A) Bilateral hand deformities. (B) Atrophy and deep central furrowing of the tongue.
Discussion
Horizontal range of motion in Moebius syndrome: BHGP versus sixth nerve palsy
The most common pattern of ocular motor deficit in Moebius syndrome is bilateral horizontal gaze palsy from pontine abducens nuclear defects, rather than abducens nerve involvement. Neurological localizations controlling conjugate horizontal eye movement are shown in Fig. 8 (Supplementary Table 2). An abducens palsy results in impaired ipsilateral abduction for saccades, smooth pursuit, and optokinetic nystagmus and is not overcome by vestibulo-ocular reflexes. A unilateral abducens nucleus lesion results in an ipsilateral horizontal gaze palsy for saccades, smooth pursuit, optokinetic nystagmus and vestibulo-ocular reflexes. Bilateral lesions result in BHGP. Adduction movements of the medial rectus may still be achieved by convergence, as convergence neurons are located in the midbrain and convergence commands do not travel in the medial longitudinal fasciculus (Mays et al., 1986; Gamlin et al., 1989). In addition to the above structures, the caudal paramedian pontine reticular formation just above the abducens nucleus houses excitatory burst neurons that project to the ipsilateral abducens nucleus to initiate saccadic eye movements (Cohen et al., 1968; Horn et al., 1995). A unilateral lesion of the paramedian pontine reticular formation results in an ipsilateral saccadic horizontal gaze palsy with slow saccades of normal or reduced range of motion, with smooth pursuit less affected and the horizontal gaze deficit often overcome by vestibulo-ocular reflexes. Optokinetic nystagmus quick-phases are generally absent in the direction of the paramedian pontine reticular formation lesion. Bilateral paramedian pontine reticular formation lesions result in bilateral horizontal saccadic gaze palsies and also cause mild slowing of vertical saccades (Henn et al., 1984).
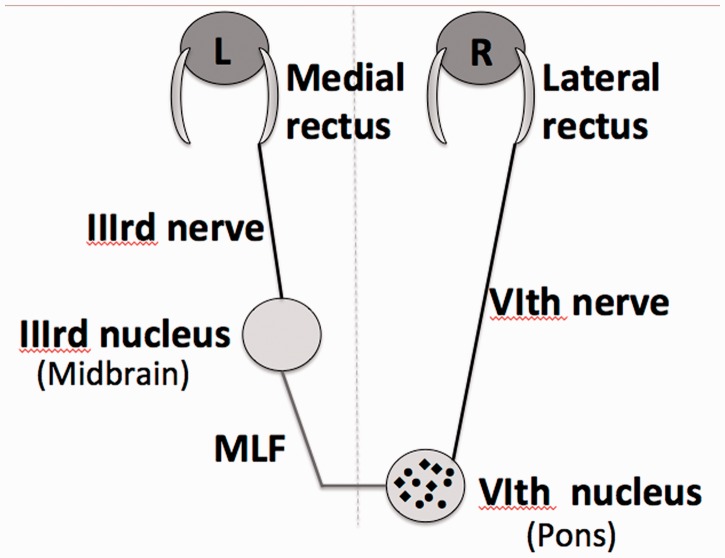
Figure 8.
Schematic diagram of structures involved in horizontal gaze, including the abducens (VIth) nucleus, the medial longitudinal fasciculus (MLF), the oculomotor (IIIrd) nucleus, the abducens (VIth) and oculomotor (IIIrd) nerves, and the lateral and medial rectus muscles. Note the two neuronal populations in the abducens nucleus. The abducens nucleus is comprised of two populations of neurons: motor neurons that project ipsilaterally to the lateral rectus and interneurons that form the medial longitudinal fasciculus. Both neuron types are present throughout the nucleus, though more interneurons are present rostrally and more motor neurons caudally.
The primary diagnostic criteria (Graefe, 1880; Möbius, 1888; Miller, 2007) for Moebius syndrome have been defined as obligate congenital, non-progressive facial weakness and limited abduction of one or both eyes. These findings are typically attributed to agenesis of the abducens (sixth) and facial (seventh) cranial nerves. However, 70% of our subjects that meet the minimal criteria of Moebius syndrome had BHGP. Only two subjects (5%) had impaired abduction in the absence of impaired adduction. In all subjects, the BHGP was present for saccades, smooth pursuit, and optokinetic nystagmus. We did not assess vestibulo-ocular reflexes; however, they have previously been reported to be absent horizontally in Moebius syndrome (Traboulsi and Maumenee, 1986). The presence of BHGP localizes to bilateral abducens nuclei. In subjects with Pattern 1, those with equally impaired abduction and adduction represent typical abducens nuclear involvement, with presumably equal dysgenesis of lateral rectus motor neurons and medial longitudinal fasciculus-destined interneurons. The subjects with Pattern 1 in whom abduction deficits were more severe than adduction deficits may have more prominent dysgenesis in the motor neuron-rich caudal portions of the abducens nuclei (Steiger and Buttner-Ennever, 1978). Supranuclear paramedian pontine reticular formation lesions are much less likely the source of BHGP, given the uniform deficit for all dynamic conjugate horizontal eye movements. Further, the clinical presence of BHGP from abducens nuclear lesions is not surprising, given pathological reports confirming abducens nuclear hypoplasia in Moebius syndrome and recent MRI demonstration of absence of the abducens and facial nuclei (Henderson, 1938; Towfighi et al., 1979; Wu et al., 2013). Indeed, patients originally described by Graefe (1880) and Möbius (Möbius, 1888; Simonsz, 2008) demonstrated BHGP and Möbius, himself, suggested that the causative structure may be the abducens nucleus. After these early accurate descriptions, weakness of adduction was largely dropped from the description and Moebius syndrome became entrenched in the literature as bilateral VI and VII nerve weakness (Thakkar et al., 1977; Meyerson and Foushee, 1978; Towfighi et al., 1979; Abramson et al., 1998; Pedraza et al., 2000; Holve et al., 2003; Traboulsi, 2007; Park et al., 2012). Attention has only recently returned to BHGP, with adduction defects reported in up to 69% (Supplementary Table 3) of patients with Moebius syndrome in recent studies (Cronemberger et al., 2001; Stromland et al., 2002; Verzijl et al., 2003; de Souza-Dias and Goldchmit, 2007; Carta et al., 2011). Several of these studies do not discuss the implications of the finding with regard to localization and to the traditionally accepted description of the syndrome; however, the abducens nucleus has been implicated (de Souza-Dias and Goldchmit, 2007; Carta et al., 2011), despite the finding of absence of convergence (Carta et al., 2011). The importance of the convergence deficit will be discussed below. In addition to studies reporting frank adduction defects, it has been suggested by some authors that the absence of a large esotropia in subjects with an abduction deficit also suggests weakness of the medial rectus, even when a discreet adduction deficit is not seen (Henderson, 1938; Metz, 1983). It is worth noting that both of our subjects with the traditionally defined Moebius ocular motor Pattern 3 had no ocular misalignment in central position, despite substantial abduction defects.
Vertical range of motion
Ten of our subjects (26%) demonstrated a reduction in vertical range of motion. Traditionally defined Moebius syndrome does not implicate abnormalities of vertical gaze. However, vertical motility deficits attributed to oculomotor nerve (cranial nerve III) involvement are reported in early descriptions of patients with congenital facial weakness and abnormalities of horizontal eye movements ascribed to Moebius syndrome (Evans, 1955; Meyerson and Foushee, 1978), including in 25% of patients in an extensive literature review (Henderson, 1938), a percentage nearly identical to that in our subjects. Even a patient originally described by Möbius demonstrated a mild reduction in the range of ocular depression in combination with BHGP (Simonsz, 2008). Pathology in a patient with complete ophthalmoplegia and congenital facial weakness revealed absence of cranial nuclei III, IV, VI and VII (Henderson, 1938). More recently, patients labelled as having deficits identical to congenital fibrosis of the extraocular muscles (CFEOM) types 1 and 2 have been reported in Moebius series (Verzijl et al., 2003; Traboulsi, 2007; Carta et al., 2011).
It is tempting to implicate the oculomotor nuclei as the localization of the vertical deficits in our subjects, given the finding that abducens nuclei rather than nerves create BHGP and given the pathologic finding described above. Clinically, a unilateral oculomotor nucleus lesion is most strongly suspected when bilateral ptosis and bilateral elevation defects are present, given a single midline caudal central subnucleus providing innervation to both levator palpebrae superioris muscles and a superior rectus subnucleus providing innervation to the contralateral superior rectus muscle. A unilateral oculomotor nucleus lesion often affects both this contralateral superior rectus innervation and the ipsilateral superior rectus innervation by affecting the decussating motor neurons from the opposite nucleus. Certainly, oculomotor nuclear lesions may be suspect in our three subjects with bilateral elevation defects in absence of depression defects; however, none of these subjects had ptosis. The affected structure in these subjects might also be the superior division of each oculomotor nerve, which innervates superior recti and levator superioris palpebrae muscles, but again none of these had ptosis. It is not possible on clinical grounds alone to localize the vertical deficits in our subjects to the oculomotor nuclei versus nerves.
It is interesting to consider whether the relative abnormality of abduction versus adduction in our subjects with ocular motor Pattern 2 may help localize their defects. Similar to subjects with Pattern 1, those with equally impaired abduction and adduction have presumably equal dysgenesis of lateral rectus motor neurons and medial longitudinal fasciculus-destined interneurons. As in Pattern 1, those in whom abduction deficits were more severe than adduction deficits may have more prominent dysgenesis in the motor neuron-rich caudal portions of the abducens nuclei (Steiger and Buttner-Ennever, 1978). What was not seen in subjects with Pattern 1, but was seen in subjects with Pattern 2, was adduction affected to a greater extent than abduction. Given the severe limitation of vertical range for elevation and depression in these subjects and the presence of bilateral ptosis in one, it is possible that medial rectus weakness as a result of decreased motor neuron innervation from oculomotor nucleus or nerve involvement contributes to the profound deficit of adduction beyond that typically seen with abducens nuclear interneuron involvement.
The relationship, if any, between our subjects with Pattern 2 who lacked ptosis and patients with heterozygous missense mutations of TUBB3, is unclear. TUBB3 mutations are reported as a cause of CFEOM3, with bilateral ptosis and restricted eye movements. Some patients have facial weakness (in overlap with our subjects), cognitive impairment, or polyneuropathy. TUBB3 encodes the neuron-specific β-tubulin isotype III, which is involved in axon guidance of commissural fibres and cranial nerves (Tischfield et al., 2010). Although CFEOM is named after congenital fibrosis of the eye muscles and some patients demonstrate positive forced duction testing, the deficits are deemed likely to be primarily because of maldevelopment of cranial nerve motor neurons (Doherty et al., 1999). MRI in one patient with CFEOM3 revealed oculomotor nerve hypoplasia and atrophy of the superior rectus, levator palpebrae superioris, and medial rectus (Tischfield et al., 2010). Ocular motor examination in CFEOM3 reveals variable degrees of limitation of vertical range of motion, minimal lateral eye movement in severely affected individuals (in overlap with our subjects), and aberrant motion of residual movements (globe retraction and palpebral fissure narrowing on lateral gaze, vertical deviations of the eyes with attempted horizontal gaze; Doherty et al., 1999). Our subjects included one individual with a confirmed genetic mutation of TUBB3. In addition, several of our subjects demonstrated aberrant innervation upon attempted horizontal gaze. Aberrant movements have been reported in Moebius series (Ghabrial et al., 1998; Verzijl et al., 2003).
Dynamic vertical eye movements and convergence
Dynamic vertical eye movements have been largely unaddressed in patients with bilateral congenital facial weakness. Vertical dynamic eye movements were abnormal in a large number of our subjects; most interestingly, even in subjects with full vertical range of motion. These findings suggest that subclinical ocular motor deficits may be present in patients with congenital facial weakness, even when eye movements appear normal upon initial assessment. Furthermore, in combination with the suggested involvement of midbrain structures described above, vertical dynamic eye movements provide further evidence to suggest that the deficits in patients with congenital facial weakness and BHGP extend beyond the lower brainstem.
Saccadic breakdown of vertical smooth pursuit in our subjects may be attributable to co-existent medial longitudinal fasciculus involvement (Baloh et al., 1978; Yee et al., 1982). Slowing of vertical saccades in our subjects is of particular interest in those with Pattern 1. This may be attributable either to co-existent bilateral paramedian pontine reticular formation or omnipause neuron involvement (Henn et al., 1984; Kaneko, 1996), or to midbrain involvement of the supranuclear centre that houses excitatory burst neurons for vertical saccades, the rostral interstitial medial longitudinal fasciculus (Horn and Buttner-Ennever, 1998). The finding of abnormal vertical optokinetic nystagmus, with generation of a slow phase only in the direction of stripe motion, in several subjects with Pattern 1 suggests the latter.
In addition to dynamic eye movement assessment in Pattern 4 subjects, careful assessment of ocular alignment is important in these patients: three of our subjects with Pattern 4 had accommodative esotropia and were previously reported to have genetically confirmed mutations in HOXB1 (Webb et al., 2012), which encodes a transcription factor important in rhombencephalic development. These individuals, specifically not characterized as Moebius syndrome because of lack of abduction deficit, are described to also have bilateral facial weakness and hearing loss (Webb et al., 2012).
The final dynamic eye movement of interest in our subjects was convergence, found to be abnormal in 66%. Convergence is uniquely known to remain intact in the presence of abducens nuclear lesions causing BHGP (Supplementary Table 2). However, the majority of our subjects with BHGP had poor to absent convergence. As convergence commands are not carried in the abducens nuclear interneurons or medial longitudinal fasciculus, its absence implies either possible co-existent involvement in supranuclear vergence centres that have been identified in the midbrain (Mays et al., 1986; Gamlin et al., 1989) or impaired relaxation of the lateral rectus muscles as a result of aberrant innervation from oculomotor axons, as occurs in Duane syndrome. Indeed, a few subjects displayed palpebral fissure narrowing and globe retraction upon horizontal gaze, but the majority with convergence deficits did not.
Additional neurological and systemic deficits
We identified neurological and systemic abnormalities in a high percentage of our subjects. Most common were self-reported imbalance, hearing loss, moderate to severe mental retardation and intellectual disability, and mild cognitive delay. Most common on examination was impaired tandem gait. Seizures, mirror movement of the limbs, weakness, and trigeminal sensory deficits were less common. It has been recognized since the early 20th century that a large percentage of patients with congenital facial weakness also have other cranial nerve or neurological deficits. Review of 61 patients with congenital facial palsy and ocular motor deficits revealed that 18% had lingual involvement and a small number had epilepsy or hearing loss (Henderson, 1938). Pathological findings in a patient with ocular motor, facial, and tongue involvement included nuclear hypoplasia in cranial nerve nuclei VI, VII and XII (Henderson, 1938). Absence of the hypoglossal eminence on brain MRI has also been reported (Pedraza et al., 2000). Developmental delay, seizures, imbalance, aberrant tearing, and hearing loss are also well reported (Meyerson and Foushee, 1978; Stromland et al., 2002; Verzijl et al., 2003; Miller et al., 2008). Although tongue, hearing and balance abnormalities implicate the lower brainstem and hindbrain, any other of the above deficits indicate more widespread injury to the CNS.
Overlap with other congenital cranial dysinnervation disorders with bilateral horizontal gaze palsy
Horizontal gaze paresis with progressive scoliosis (HGPPS) is a disorder caused by mutations in ROBO3, which encodes a transmembrane protein involved in axonal midline crossing that is expressed in hindbrain and spinal cord axons during embryogenesis (Jen et al., 2004; Chan et al., 2006). It is typified clinically by progressive scoliosis and BHGP for smooth pursuit, saccades, optokinetic nystagmus, and vestibulo-ocular reflexes (Bosley et al., 2005)—similar to the BHGP seen in many of our subjects, though none of our subjects had scoliosis or the horizontal pendular nystagmus typical of HGPPS and facial weakness is generally absent in HGPPS. Previous eye movement studies in HGPPS revealed the presence of BHGP affecting smooth pursuit, saccades, optokinetic nystagmus and vestibulo-ocular reflexes; variable convergence from normal to absent; saccadic smooth pursuit in the vertical direction, and impaired vertical optokinetic nystagmus (Yee et al., 1982; Bosley et al., 2005). Abnormalities of vertical smooth pursuit and optokinetic nystagmus were attributed to co-existent medial longitudinal fasciculus involvement (Yee et al., 1982). Though patients with HGPPS undoubtedly clinically differ substantially from our subjects, it is hard to deny that the BHGP and vertical dynamic eye movement abnormalities are similar.
Mice with ROBO3 deletions were found to have poor horizontal eye movements similar to humans with HGPPS (Renier et al., 2010). The authors showed that the genetic mutation, although not affecting the pathfinding and formation of abducens cranial nerve neurons, disrupted the formation of the abducens-oculomotor internuclear commissural projection (the homologue of the medial longitudinal fasciculus in humans). It was concluded that absence of this structure, with additional possible paramedian pontine reticular formation non-decussation, explains both the medial and lateral impaired eye movement in patients with HGPPS (Renier et al., 2010; Nugent et al., 2012). However, the aetiology for BHGP is not readily attributable to the above explanation, as absent bilateral medial longitudinal fasciculi would result only in impaired adduction of both eyes with intact abduction and the excitatory burst neurons comprising the paramedian pontine reticular formation project ipsilaterally to the abducens nucleus for saccade generation and do not decussate.
A second disorder, Athabascan brainstem dysgenesis syndrome, has been described as distinct from Moebius syndrome because of the presence of BHGP rather than abducens nerve involvement and due to the presence of deafness and central hypoventilation (Holve et al., 2003). Developmental delay, poor facial movement, seizures, and cerebellar dysfunction were seen in some patients. The patients described have features similar to those seen in many of our subjects; however, screening of a 40 proband sample of 40 subjects with Moebius syndrome failed to reveal mutations in HOXA1 that have been identified in subjects with Athabascan brainstem dysgenesis syndrome (Rankin et al., 2010). Again, although patients with this disorder vary clinically and genetically from our subjects, it is difficult to ignore the similarities in the eye movement findings.
HGPPS and Athabascan brainstem dysgenesis share—in common with the majority of our subjects—an indistinguishable BHGP. In all of these disorders, BHGP is the most common and dominant eye movement disorder and localizes clinically to the abducens nuclei.
Conclusion
Ocular motor findings in patients with congenital facial weakness can be phenotypically categorized into four ‘patterns’: BHGP (Pattern 1); BHGP with vertical range impairments (Pattern 2); impaired abduction (Pattern 3); and full ocular motor range (Pattern 4). Patients with the first three patterns fulfill the criteria for the diagnosis of Moebius syndrome; whereas those with Pattern 4 do not meet criteria, but may have other ocular motor deficits. Our study indicates that further subcategorization may be possible based on concurrent abnormalities of dynamic eye movement. A weakness of the current study is the lack of quantified ocular motor recording data to substantiate the clinical ocular motor dynamic findings, though certainly this study will prompt further investigation in patients with congenital facial weakness. Our research group is in the process of collecting and analysing such quantitative ocular motor recording data. Additional questions, such as whether the vertical saccades are truly slowed or whether they are multi-step and hypometric, will need to be addressed by quantitative recordings.
The most common eye movement abnormality in Moebius syndrome is BHGP, localizing clinically to bilateral abducens nuclei. Many of these patients do seem to have a developmental disorder of the entire lower brainstem rather than just the cranial nerve nuclei (Verzijl et al., 2003); however, clinical extension even beyond the lower brainstem and cerebellum are common. The considerable similarities and differences among the ocular motor deficits and other clinical features in a number of the congenital cranial dysinnervation disorders (Holve et al., 2003; Traboulsi, 2004; Tischfield et al., 2005) underscore the need for further detailed study of ocular motility to facilitate ocular motor localization and future detection of underlying genetic diagnosis.
Supplementary Material
Acknowledgements
We thank the Moebius Syndrome Foundation for their support of our research and for assisting with access to subjects for research involvement.
Glossary
Abbreviations
- BHGP
bilateral horizontal gaze palsy
- CFEOM
congenital fibrosis of the extraocular muscles
- HGPPS
horizontal gaze paresis with progressive scoliosis
Funding
This manuscript was supported in part by The Moebius Syndrome Foundation (B.W. and E.W.J.). Dr. Webb is funded by NIH training grant NIH T32 GM082773-05.
Supplementary material
Supplementary material is available at Brain online.
References
- Abramson DL, Cohen MM, Jr, Mulliken JB. Mobius syndrome: classification and grading system. Plast Reconstr Surg. 1998;102:961–7. doi: 10.1097/00006534-199809040-00004. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Yee RD, Honrubia V. Internuclear ophthalmoplegia. II. Pursuit, optokinetic nystagmus, and vestibulo-ocular reflex. Arch Neurol. 1978;35:490–3. doi: 10.1001/archneur.1978.00500320010003. [DOI] [PubMed] [Google Scholar]
- Bavinck JN, Weaver DD. Subclavian artery supply disruption sequence: hypothesis of a vascular etiology for Poland, Klippel-Feil, and Mobius anomalies. Am J Med Genet. 1986;23:903–18. doi: 10.1002/ajmg.1320230405. [DOI] [PubMed] [Google Scholar]
- Bosley TM, Salih MA, Jen JC, Lin DD, Oystreck D, Abu-Amero KK, et al. Neurologic features of horizontal gaze palsy and progressive scoliosis with mutations in ROBO3. Neurology. 2005;64:1196–203. doi: 10.1212/01.WNL.0000156349.01765.2B. [DOI] [PubMed] [Google Scholar]
- Carta A, Mora P, Neri A, Favilla S, Sadun AA. Ophthalmologic and systemic features in mobius syndrome an italian case series. Ophthalmology. 2011;118:1518–23. doi: 10.1016/j.ophtha.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Chan WM, Traboulsi EI, Arthur B, Friedman N, Andrews C, Engle EC. Horizontal gaze palsy with progressive scoliosis can result from compound heterozygous mutations in ROBO3. J Med Genet. 2006;43:e11. doi: 10.1136/jmg.2005.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S, Balasubramanian R, Chan WM, Kang PB, Andrews C, Webb BD, et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal beta-tubulin isotype 3. Brain. 2013;136(Pt 2):522–35. doi: 10.1093/brain/aws345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Komatsuzaki A, Bender MB. Electrooculographic syndrome in monkeys after pontine reticular formation lesions. Arch Neurol. 1968;18:78–92. doi: 10.1001/archneur.1968.00470310092008. [DOI] [PubMed] [Google Scholar]
- Cronemberger MF, de Castro Moreira JB, Brunoni D, Mendonca TS, Alvarenga EH, Rizzo AM, et al. Ocular and clinical manifestations of Mobius' syndrome. J Pediatr Ophthalmol Strabismus. 2001;38:156–62. doi: 10.3928/0191-3913-20010501-09. [DOI] [PubMed] [Google Scholar]
- de Souza-Dias CR, Goldchmit M. Further considerations about the ophthalmic features of the Mobius sequence, with data of 28 cases. Arq Bras Oftalmol. 2007;70:451–7. doi: 10.1590/s0004-27492007000300012. [DOI] [PubMed] [Google Scholar]
- Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty EJ, Macy ME, Wang SM, Dykeman CP, Melanson MT, Engle EC. CFEOM3: a new extraocular congenital fibrosis syndrome that maps to 16q24.2-q24.3. Invest Ophthalmol Vis Sci. 1999;40:1687–94. [PubMed] [Google Scholar]
- Evans PR. Nuclear agenesis. Mobius syndrome: the congenital facial diplegia syndrome. Arch Dis Child. 1955;30:237–42. doi: 10.1136/adc.30.151.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Gnadt JW, Mays LE. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. J Neurophysiol. 1989;62:70–81. doi: 10.1152/jn.1989.62.1.70. [DOI] [PubMed] [Google Scholar]
- Ghabrial R, Versace P, Kourt G, Lipson A, Martin F. Mobius' syndrome: features and etiology. J Pediatr Ophthalmol Strabismus. 1998;35:304–11. doi: 10.3928/0191-3913-19981101-04. quiz 27–8. [DOI] [PubMed] [Google Scholar]
- Graefe A. In: Handbuch der gesammten Augenheilkunde. Graefe A, Saemisch T, editors. Leipzig: Engelmann; 1880. p. 60. [Google Scholar]
- Gutowski NJ, Bosley TM, Engle EC. 110th ENMC International Workshop: the congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25-27 October, 2002. Neuromuscul Disord. 2003;13:573–8. doi: 10.1016/s0960-8966(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Henderson JL. The congenital facial diplegia syndrome: clinical features, pathology, and aetiology. Brain. 1938;62:381–403. [Google Scholar]
- Henn V, Lang W, Hepp K, Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain. 1984;107 (Pt 2):619–36. doi: 10.1093/brain/107.2.619. [DOI] [PubMed] [Google Scholar]
- Holve S, Friedman B, Hoyme HE, Tarby TJ, Johnstone SJ, Erickson RP, et al. Athabascan brainstem dysgenesis syndrome. Am J Med Genet A. 2003;120A:169–73. doi: 10.1002/ajmg.a.20087. [DOI] [PubMed] [Google Scholar]
- Horn AK, Buttner-Ennever JA. Premotor neurons for vertical eye movements in the rostral mesencephalon of monkey and human: histologic identification by parvalbumin immunostaining. J Comp Neurol. 1998;392:413–27. [PubMed] [Google Scholar]
- Horn AK, Buttner-Ennever JA, Suzuki Y, Henn V. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol. 1995;359:350–63. doi: 10.1002/cne.903590212. [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–13. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko CR. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J Neurophysiol. 1996;75:2229–42. doi: 10.1152/jn.1996.75.6.2229. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–21. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- Metz HS. Saccadic velocity measurements in strabismus. Trans Am Ophthalmol Soc. 1983;81:630–92. [PMC free article] [PubMed] [Google Scholar]
- Meyerson MD, Foushee DR. Speech, language, and hearing in Moebius syndrome: a study of 22 patients. Develop Med Child Neurol. 1978;20:357–65. doi: 10.1111/j.1469-8749.1978.tb15225.x. [DOI] [PubMed] [Google Scholar]
- Miller G. Neurological disorders. The mystery of the missing smile. Science. 2007;316:826–7. doi: 10.1126/science.316.5826.826. [DOI] [PubMed] [Google Scholar]
- Miller MT, Stromland K, Ventura L. Congenital aberrant tearing: a re-look. Trans Am Ophthalmol Soc. 2008;106:100–15. discussion 115–6. [PMC free article] [PubMed] [Google Scholar]
- Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int J Dev Neurosci. 2005;23:201–19. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Möbius PJ. Ueber angeborene doppelseitige Abducens-Facialis-Lahmung. Munch Med Wochenschr. 1888;35:91–4. [Google Scholar]
- Nugent AA, Kolpak AL, Engle EC. Human disorders of axon guidance. Curr Opin Neurobiol. 2012;22:837–43. doi: 10.1016/j.conb.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Kim JH, Hwang JM. Coexistence of Mobius syndrome and Duane's retraction syndrome. Graefes Arch Clin Exp Ophthalmol. 2012;250:1707–9. doi: 10.1007/s00417-011-1816-4. [DOI] [PubMed] [Google Scholar]
- Parker DL, Mitchell PR, Holmes GL. Poland-Mobius syndrome. J Med Genet. 1981;18:317–20. doi: 10.1136/jmg.18.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza S, Gamez J, Rovira A, Zamora A, Grive E, Raguer N, et al. MRI findings in Mobius syndrome: correlation with clinical features. Neurology. 2000;55:1058–60. doi: 10.1212/wnl.55.7.1058. [DOI] [PubMed] [Google Scholar]
- Rankin JK, Andrews C, Chan WM, Engle EC. HOXA1 mutations are not a common cause of Mobius syndrome. J Aapos. 2010;14:78–80. doi: 10.1016/j.jaapos.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, et al. Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsz HJ. Historical perspective: first description of the Moebius syndrome. Strabismus. 2008;16:3. doi: 10.1080/09273970801891446. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Buttner-Ennever J. Relationship between motoneurons and internuclear neurons in the abducens nucleus: a double retrograde tracer study in the cat. Brain Res. 1978;148:181–8. doi: 10.1016/0006-8993(78)90387-6. [DOI] [PubMed] [Google Scholar]
- Stromland K, Sjogreen L, Miller M, Gillberg C, Wentz E, Johansson M, et al. Mobius sequence–a Swedish multidiscipline study. Eur J Paediatr Neurol. 2002;6:35–45. doi: 10.1053/ejpn.2001.0540. [DOI] [PubMed] [Google Scholar]
- Thakkar N, O'Neil W, Duvally J, Liu C, Ambler M. Mobius syndrome dueto brain stem tegmental necrosis. Arch Neurol. 1977;34:124–6. doi: 10.1001/archneur.1977.00500140078018. [DOI] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Bosley TM, Salih MA, Alorainy IA, Sener EC, Nester MJ, et al. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–7. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Marks K, Palmer E, Vannucci R. Mobius syndrome. Neuropathologic observations. Acta Neuropathol. 1979;48:11–7. doi: 10.1007/BF00691785. [DOI] [PubMed] [Google Scholar]
- Traboulsi EI. Congenital abnormalities of cranial nerve development: overview, molecular mechanisms, and further evidence of heterogeneity and complexity of syndromes with congenital limitation of eye movements. Trans Am Ophthalmol Soc. 2004;102:373–89. [PMC free article] [PubMed] [Google Scholar]
- Traboulsi EI. Congenital cranial dysinnervation disorders and more. J Aapos. 2007;11:215–7. doi: 10.1016/j.jaapos.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Traboulsi EI, Maumenee IH. Extraocular muscle aplasia in Moebius syndrome. J Pediatr Ophthalmol Strabismus. 1986;23:120–2. doi: 10.3928/0191-3913-19860501-05. [DOI] [PubMed] [Google Scholar]
- Ventura BV, Miller MT, Danda D, Carta A, Brandt CT, Ventura LO. Profile of ocular and systemic characteristics in Mobius sequence patients from Brazil and Italy. Arq Bras Oftalmol. 2012;75:202–6. doi: 10.1590/s0004-27492012000300011. [DOI] [PubMed] [Google Scholar]
- Verzijl HT, van der Zwaag B, Cruysberg JR, Padberg GW. Mobius syndrome redefined: a syndrome of rhombencephalic maldevelopment. Neurology. 2003;61:327–33. doi: 10.1212/01.wnl.0000076484.91275.cd. [DOI] [PubMed] [Google Scholar]
- Webb BD, Shaaban S, Gaspar H, Cunha LF, Schubert CR, Hao K, et al. HOXB1 founder mutation in humans recapitulates the phenotype of Hoxb1-/- mice. Am J Hum Genet. 2012;91:171–9. doi: 10.1016/j.ajhg.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Man F, Jiao Y, Xian J, Wang Y, Wang Z. Magnetic resonance imaging findings in sporadic Mobius syndrome. Chin Med J. 2013;126:2304–7. [PubMed] [Google Scholar]
- Yamada K, Andrews C, Chan WM, McKeown CA, Magli A, de Berardinis T, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1) Nat Genet. 2003;35:318–21. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- Yamada K, Chan WM, Andrews C, Bosley TM, Sener EC, Zwaan JT, et al. Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3) Invest Ophthalmol Vis Sci. 2004;45:2218–23. doi: 10.1167/iovs.03-1413. [DOI] [PubMed] [Google Scholar]
- Yee RD, Duffin RM, Baloh RW, Isenberg SJ. Familial, congenital paralysis of horizontal gaze. Arch Ophthalmol. 1982;100:1449–52. doi: 10.1001/archopht.1982.01030040427011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.