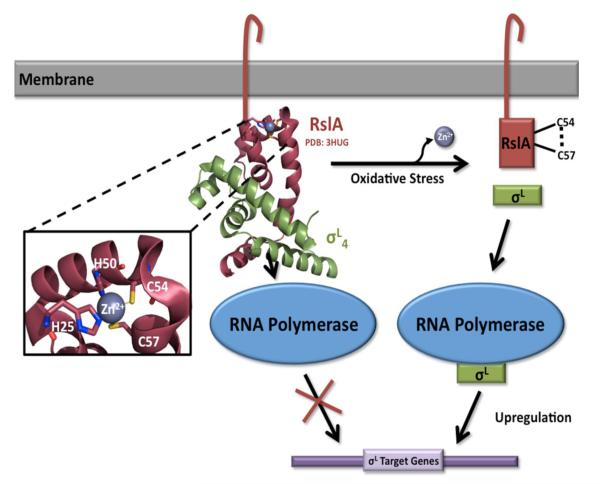
Figure 3.
The mechanism of SigL regulation. The membrane-associated anti-σ factor, RslA, contains a conserved cytosolic ZAS (HXXXCXXC) motif, which coordinates a structural Zn2+ ion under reducing conditions. SigL function is inhibited through complex formation occluding SigL’s predicted -35 promoter binding domain (PDB code 3HUG). Upon exposure to oxidative stress, the two cysteines of the CXXC motif (Cys54 and Cys57) form a disulfide bond, releasing the Zn2+ ion and leading to a conformational change in RslA. This conformational change results in the dissociation of the RslA/SigL complex, allowing SigL to interact with RNA polymerase and upregulate target genes.