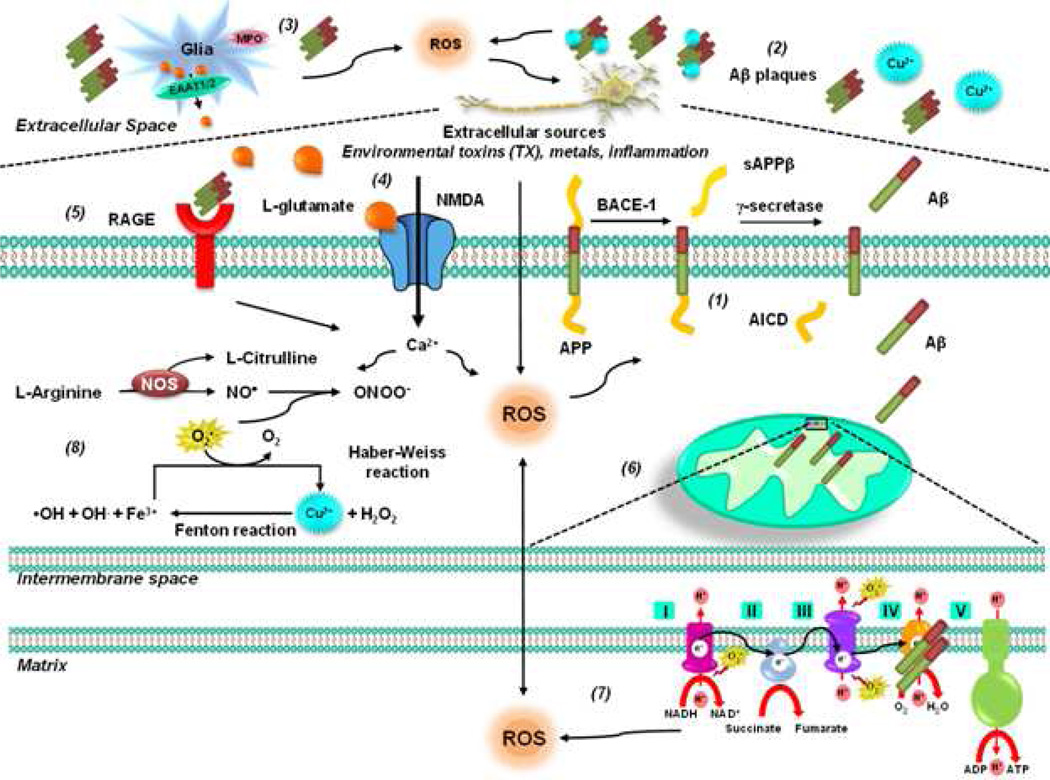
Figure 1. Oxidative stress in AD.
The amyloid cascade hypothesis suggests that deposition of Aβ triggers neuronal dysfunction and death in the brain. APP is an integral membrane protein concentrated in the synapses of neurons. (1) Amyloidogenic processing is initiated by β-secretase beta-site amyloid precursor protein–cleaving enzyme 1 (BACE-1), releasing a shortened sAPP. The C99 fragment is a γ-secretase substrate, generating Aβ and AICD. PSEN1 and PSEN2 mutations affect concentrations of Aβ1–42 because presenilin proteins form part of γ secretase, which cleaves APP to produce Aβ. (2) Soluble Aβ is prone to aggregation. In addition, Aβ interacts with several metal ions affecting its solubility and leading to fibrillization and cellular toxicity. The Aβ-metal complex triggers ROS production. (3) Activation of glial cells has also been proposed to contribute to ROS formation via MPO. (4) In addition, accumulation of extracellular levels of the exicitatory amino acid glutamate leads to perturbations in neuronal Ca2+ homeostasis that have the potential to trigger mitochondrial dysfunction and ROS formation. (5) The RAGE has also been proposed to mediate Aβ's pro-oxidant effects. (6) Aβ has been shown to be transported into mitochondria via the outer membrane (TOM) complex and localized at the mitochondrial cristae. (7) In the mitochondria, Aβ inhibits mitochondrial cytochrome oxidase (complex IV) and the key Krebs-cycle enzymes (α-ketoglutarate and pyruvate dehydrogenase) impairing ETC, ATP production, oxygen consumption, and mitochondrial membrane potential. Dysfunctional mitochondria produce ROS and mtDNA oxidative damage. Conversely, mitochondrial ROS also trigger increased Aβ production. (8) Aβ induces an increase in iNOS expression and NO• ONOO− generation in glial cells, whereas it inhibits the activity of nNOS and eNOS in neuronal-like cells.