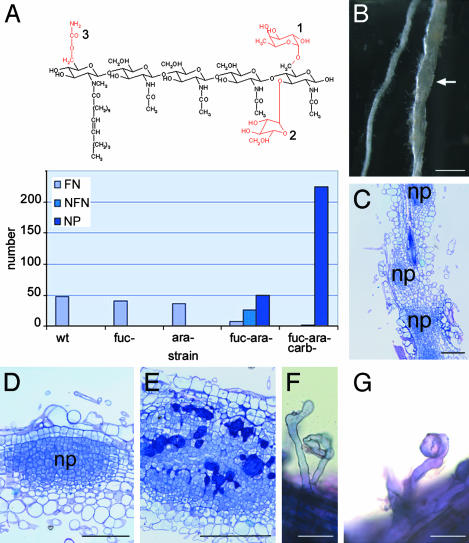
Fig. 3.
Nod factor structure requirements for root hair infection on S. rostrata. (A) Structure of the main Nod factor produced by A. caulinodans ORS571. Substitutions in red are the chemical groups that are removed in the different mutant strains used in the experiment. 1, fucosyl moiety; 2, arabinosyl moiety; and 3, carbamoyl moiety. The histogram shows the number of functional nodules (FN), nonfunctional nodules (NFN), and nodule primordia (NP) caused by zone I infection on vermiculite-grown roots, 7 DPI, infected with wild-type ORS571 or several mutants. Fuc-, ara-, and carbindicate that the mutant strain produced Nod factors lacking the fucosyl, arabinosyl, or carbamoyl moiety, respectively. Because of the variable nodulation efficiency of different plants, the results presented are the sum of lateral root base or zone I nodules formed on 12 plants. (B) Vermiculite-grown roots infected with ORS571(1.31U-ΩK), 7 DPI. Arrow indicates swollen root. (C) Toluidine blue-stained section through a swollen root reveals that the swollen root appearance is due to the presence of a sequence of nodule primordia. (D) Toluidine blue-stained sections through a nodule primordium grown after infection with ORS571(1.31U-ΩK). (E) Toluidine blue-stained section of a colonized nodule primordium grown after infection with ORS571(1.31U-ΩK). Bacteria are seen in large intercellular spaces between the primordial cells. (F and G) Methylene blue-stained deformed and curled root hairs, respectively, of vermiculite-grown S. rostrata roots after infection with ORS571(1.31U-ΩK). Abbreviations, see Fig. 2 legend. (Bars = 1 cm in B and 100 μm in C–G.)