Abstract
Background
The American Heart Association (AHA) recently developed the Cardiovascular Health Index (CVHI), a health metric consisting of 7 modifiable risk factors. The relationship of the CVHI with preclinical markers, such as carotid intima‐media thickness (CIMT) has not been assessed.
Methods
We examined 490 male monozygotic and dizygotic twins without overt cardiovascular disease. CIMT was measured using B‐mode ultrasonography. Each of the 7 CVHI components (blood pressure, fasting glucose, total cholesterol, body mass index, physical activity, healthy diet, and smoking) was given a point score of 0, 1, or 2 to represent poor, intermediate, or ideal health, respectively. A CVHI summation score was computed (range 0 to 14) and categorized as inadequate (0 to 4), average (5 to 9), or optimum (10 to 14) cardiovascular health. Mixed‐model regression was used to examine the association of the CVHI with CIMT.
Results
The mean age of the twins was 55.4 years, and 61% were monozygotic. The mean CIMT was 0.75 (±0.11) mm and the mean CVHI score was 7.7 (±2.1). There was an inverse correlation between CVHI and CIMT (Spearman r=−0.22, P<0.01). For every 5‐unit increase in overall CVHI score (indicating better cardiovascular health category), CIMT decreased by 0.045 mm (P<0.001) after adjusting for demographic variables and other confounders. Within monozygotic twin pairs, a 5‐unit increment in CVHI score was associated with a 0.05 mm lower CIMT (P<0.001).
Conclusions
The CVHI is independently associated with CIMT and the association is not confounded by shared genetic and other familial factors.
Keywords: epidemiology, prevention, risk factors
Introduction
Epidemiological studies have suggested the importance of a global risk profile in the prediction and prevention of cardiovascular disease.1 Most cardiovascular disease events are preventable by optimizing ≥1 cardiovascular risk factors.2–5 The American Heart Association and the American Stroke Association (AHA/ASA) proposed a new public health metric: the Cardiovascular Health Index (CVHI), which is a measure of cardiovascular risk factors and can be followed over time.6–7 This index emphasizes primary prevention by defining goals for health factors and behaviors that comprise the definition of “ideal” cardiovascular health. The CVHI has 7 components, which include 3 health factors (blood pressure, fasting glucose, and total cholesterol) and 4 health behaviors (body mass index [BMI], physical activity, healthy diet, and smoking) and classifies each of them into ideal, intermediate, and poor levels. For disease prevention and improving cardiovascular health in the general population, early detection of CVD must be emphasized as a goal.
Carotid Intima‐Media Thickness (CIMT), a preclinical marker for cardiovascular disease, is a quantitative index for evaluating the severity and progression of atherosclerosis and for the prediction of coronary heart disease and stroke.8–14 CIMT is also associated with several cardiovascular risk factors including blood pressure, dyslipidemia, and health behaviors such as smoking and physical activity. However, the relationship of CIMT with measures of CVD health as part of the CVHI has not been demonstrated.15–17 In adulthood, many health behaviors and other CVD risk factors, are influenced by the familial environment experienced during childhood and adolescence, as well as by genetic factors.18–19 Therefore, studies of CIMT and cardiovascular risk factors may be subtly confounded by exposures or behaviors that are shared by members of the same family.20–22 Our objective in this study was to assess the relationship between the CVHI and CIMT while accounting for the influence of unmeasured familial factors (including genetic and early environment factors) in a twin sample. We hypothesized that people with lower CVHI (indicating poorer cardiovascular health) would have increased CIMT. Furthermore, we hypothesized that this relationship is continuous and graded across all levels of the CVHI and not confounded by shared genetic and other familial factors.
Methods
Study Population
The Emory Twin Study includes samples recruited in 2 companion studies: the Twins Heart Study (THS) and the Stress and Vascular Evaluation in Twins (SAVEIT). The purpose of these studies was to elucidate the role of psychological, behavioral, and biologic risk factors for subclinical cardiovascular disease in twins. Both studies recruited randomly selected samples of middle‐aged male monozygotic (MZ) and dizygotic (DZ) twin pairs (who were raised in the same household) from the Vietnam Era Twin (VET) Registry. The VET Registry includes 7369 male‐male twin pairs of whom both twins served in the US military during the time of the Vietnam War.23 Both studies followed identical procedures, measurements, and protocols. THS enrolled 180 twin pairs between 2002 and 2006 and SAVEIT enrolled 127 twin pairs between 2005 and 2010 as previously described.24–26 We excluded participants with a history of coronary artery disease and stroke. We also excluded a few duplicate twin pairs who had either participated in both studies or were missing information on CIMT. Each twin in a recruited pair was examined at the same time at the Emory University General Clinical Research Center, and all data collection including medical history and physical examination and blood tests, occurred during a 24‐hour admission under controlled conditions. Information on sociodemographic and lifestyle factors was collected using standardized questionnaires. Depressive symptoms were ascertained by using the Beck Depression Inventory‐II, which has satisfactory test‐retest reliability and internal consistency.27–28 Information on current use of medications was also collected. Both studies were approved by the Emory Institutional Review Board, and all twins gave informed consent. Zygosity information by means of DNA typing was available for all twin pairs.
Cardiovascular Health Index
Components of the CVHI include blood pressure, fasting glucose, total cholesterol, body mass index, physical activity, diet, and smoking. Each CVHI component was given a point score of 0, 1, or 2 to represent poor, intermediate, or ideal health, respectively, as per predefined categories. Based on the sum of all 7 CVHI components, an overall CVHI score, ranging from 0 to 14, was categorized as inadequate (0 to 4), average (5 to 9), or optimum (10 to 14) cardiovascular health (Table 1). Systolic blood pressure and diastolic blood pressure were measured by mercury sphygmomanometer on the right arm with the subject in a sitting position after 10 minutes of rest. The average of 2 measurements 5 minutes apart was used in the statistical analyses. Venous blood samples were drawn for the measurement of glucose and lipid profile (total cholesterol) after an overnight fast. The Emory Lipid Research Laboratory, a participant in the Centers for Disease Control/National Heart, Lung and Blood Institute Lipid Standardization Program, performed all analyses from freshly isolated ethylenediaminetetraacetic acid (EDTA) plasma. Glucose levels were measured on the Beckman CX7 chemistry autoanalyzer. Body mass index was calculated from height and weight measurement of participants. Physical activity was determined by means of a modified version of the Baecke Questionnaire of habitual physical activity.29 We used tertiles of the cumulative Baecke score to classify individuals into poor, intermediate, and ideal levels of physical activity. To measure the specific score for diet we used the DASH (Dietary Approaches to Stop Hypertension) diet score, which is endorsed by AHA and has been linked to diminished risk of CHD and stroke.30–31 We constructed the DASH score according to the method proposed by Fung et al used in several epidemiological studies.32 The DASH score is calculated based on 8 food items (fruits, vegetables, nuts and legumes, low fat dairy products, whole grains, sodium, sweetened beverages, red and processed meats) and on the following principles: (1) high intake of fruits, vegetables, nuts and legumes, low‐fat dairy products, and whole grains are beneficial for human health and receive high scores; (2) high intake of sodium, sweetened beverages, and red and processed meats are harmful and deserve lower scores. For each of the above 8 food groups, we categorized study subjects into quintiles (assigned 1 to 5 points) according to their individual food intake component scores. Scoring by quintile helped to reduce the potential for misclassification. The scores for each food group were then summed to yield an overall score ranging from 8 to 40, where higher scores represent greater adherence to the DASH diet. The cumulative score was grouped into tertiles to classify individuals with ideal, intermediate, and poor diets. Cigarette smoking was classified into current smoker (poor); quit in the past year (intermediate); and never smoker or quit >1 year ago (ideal).
Table 1.
Cardiovascular Health Index Definition and Scoring
| Health Metric | Levels | Score | Definition |
|---|---|---|---|
| Blood pressure | Ideal | 2 | <120/<80 mm Hg, without antihypertensive medication |
| Intermediate | 1 | SBP 120 to 139 or DBP 80 to 89 mm Hg or treated with antihypertensive to <120/<80 mm Hg | |
| Poor | 0 | SBP ≥140 or DBP ≥90 mm Hg | |
| Fasting glucose | Ideal | 2 | <100 mg/dL, without antidiabetes medication |
| Intermediate | 1 | 100 to 125 mg/dL or treated with antidiabetes to <100 mg/dL | |
| Poor | 0 | ≥126 mg/dL | |
| Total cholesterol | Ideal | 2 | <200 mg/dL, without lipid lowering medication |
| Intermediate | 1 | 200 to 239 mg/dL or treated to <200 mg/dL | |
| Poor | 0 | ≥240 mg/dL | |
| Body mass index | Ideal | 2 | <25 kg/m2 |
| Intermediate | 1 | 25 to 29.99 kg/m2 | |
| Poor | 0 | ≥30 kg/m2 | |
| Physical activity* | Ideal | 2 | 4 or more times per week of intense physical activity |
| Intermediate | 1 | 1 to 3 times per week of intense physical activity | |
| Poor | 0 | No physical activity | |
| Healthy diet score* | Ideal | 2 | 4 to 5 components |
| Intermediate | 1 | 2 to 3 components | |
| Poor | 0 | 0 to 1 components | |
| Smoking | Ideal | 2 | Never or quit >12 months |
| Intermediate | 1 | Former, quit ≤12 months | |
| Poor | 0 | Current |
DASH indicates Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Modified for Emory Twin Study and based on tertiles of Baecke Physical Activity Questionnaire.
Modified for Emory Twin Study and based on tertiles of DASH diet score.
Carotid Intima Media Thickness
CIMT was measured using high resolution B‐mode ultrasonography with standard techniques.33–34 Briefly, CIMT was quantified both on the near and far wall at the distal 1.0 cm of both the left and right common carotid arteries proximal to the bifurcation. For each segment, the sonographer used multiple different scanning angles to identify the longitudinal image of CIMT showing the maximum CIMT. At least 10 pictures for each segment were stored digitally, and measurements were made offline using semiautomated computerized analytical software (Carotid Tools, MIA Inc) by one observer blinded to other twin data. Of the stored images, the one with maximum thickness was selected, and CIMT measured, for each segment. Average values of the CIMT of each of the 4 segments (right near and far walls, and left near and far walls) were used as the CIMT values for each twin in the analysis (total mean of maximum CIMT). In order to minimize variability, the same technician did CIMT measurements throughout the study, and the same equipment and analytical software was used to measure CIMT for all the twin participants. In our laboratory, the mean absolute difference in CIMT measured in 7 subjects in whom 2 carotid artery examinations were performed 3 days apart, was 0.03 (±0.02) mm. The mean difference in 2 successive readings of the same 10 segments of CIMT was 0.02 (±0.02) mm with a Pearson correlation coefficient of 0.93.
Statistical Analyses
Continuous variables were described as mean±SD and categorical variables as frequencies (percent). We examined baseline demographic characteristics, cardiovascular health factors, and behaviors and medications across CVHI categories, treating the twins as individuals. We also compared individual CVHI components across CIMT categories (greater or less than the median of 0.75) and with CIMT as a continuous score while accounting for correlated data using mixed models or generalized estimating equation (GEE) models. In additional analyses, we examined the Spearman correlation between CVHI and CIMT and the relation between CIMT and number of ideal health factors.
Next, we analyzed the relationship between CIMT and CVHI categories using mixed model regression analysis adapted for twin studies.35 We fitted mixed models for twins that allowed for partitioning within and between pair differences in the dependent variable as a function of the independent variables. In these models, the within‐pair term was defined as a difference of at least 1 point in the CVHI between the 2 brothers. This within‐pair analysis by design takes into account shared genetic and many early environmental factors. Within‐pair analysis was further stratified by zygosity to determine whether the relationship between CVHI and CIMT was different between MZ and DZ twins. Monozygotic twins share 100% of their genetic material, and therefore differences between MZ twins are controlled for genetic factors. Dizygotic twins share on average 50% of genes and differences between the twins are only partially controlled for shared genetic factors. Shared genetic factors would be implicated if the within‐pair difference in CIMT in CVHI‐discordant pairs were smaller in MZ than in DZ pairs. Potential multicollinearity was investigated using condition indices and variance decomposition proportions. All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute Inc). Significance level was set at 0.05, 2‐sided.
Results
From the initial sample of 307 twin pairs, 40 twin pairs were excluded because of history of coronary artery disease and 9 twin pairs were excluded because of history of stroke. We also excluded a few duplicate twin pairs who participated in both studies and twins missing information on CIMT (13 twin pairs). We had complete data on CIMT in 245 twin pairs or 490 twins. The mean age of participants was 55.4 (±3.12) years and 96% were white; 61% were MZ and 39% were DZ twins, a distribution that reflects that of the entire Vietnam Era Twin Registry. The mean CIMT was 0.75 (±0.11) mm and the mean CVHI score was 7.7 (±2.06). Overall, 18% of participants had optimum, 77% had average, and 5% had inadequate cardiovascular health. The age‐adjusted prevalence of ideal CVHI components ranged from 15% for BMI to 75% for smoking (Figure 1). Less education and unemployment were associated with inadequate cardiovascular health (Table 2).
Figure 1.
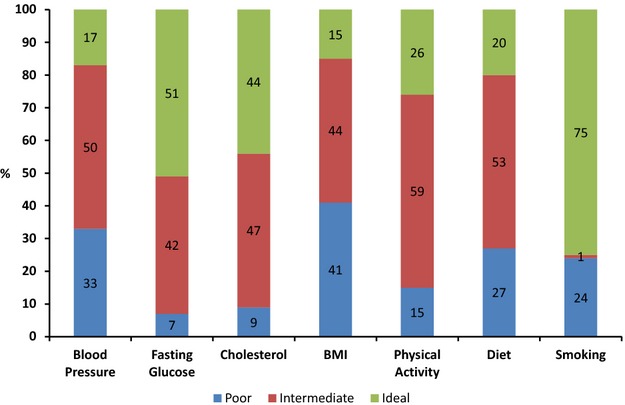
Cardiovascular health profile of Emory Twin Study participants (n=490). BMI indicates body mass index.
Table 2.
Distribution of Covariates of Emory Twin Study Participants (n=490) by Cardiovascular Health Categories
| Covariates | Cardiovascular Health Index (CVHI) Category | P Value | ||
|---|---|---|---|---|
| Optimum (CVHI=10 to 14) N=89 | Average (CVHI=5 to 9) N=375 | Inadequate (CVHI=0 to 4) N=26 | ||
| Age, y | 54.9 | 55.4 | 55.5 | 0.29 |
| Systolic blood pressure, mm Hg | 121.6 | 131.4 | 140.0 | <.001 |
| Low‐density lipoprotein–cholesterol | 116.4 | 123.4 | 131.5 | 0.07 |
| Body mass index, kg/m2 | 26.7 | 30.0 | 32.3 | <0.001 |
| Plasma glucose, mg/dL | 93.5 | 103.5 | 119.8 | <0.001 |
| Current employment, % | 95.7 | 82.4 | 62.9 | <0.001 |
| Number of alcoholic drinks/week | 4.8 | 5.2 | 4.9 | 0.68 |
| College education, % | 82.9 | 67.6 | 61.5 | 0.008 |
| Current smoking, % | 0 | 40 | 75 | <0.001 |
| Depression, % | 20.2 | 26.7 | 28.0 | 0.4 |
| Medications, % | ||||
| Thiazide | 1.2 | 7.2 | 7.7 | 0.09 |
| Beta‐blocker | 4.5 | 10.6 | 7.7 | 0.19 |
| Aspirin | 18.7 | 75.8 | 5.4 | 0.08 |
| Angiotensin converting enzyme inhibitors | 5.6 | 14.1 | 23.1 | 0.05 |
| Statin | 9.0 | 23.2 | 1.6 | 0.08 |
| Anti‐depressant | 1.2 | 3.5 | 0 | 0.3 |
| Diabetes | 2.3 | 5.1 | 5.9 | 0.64 |
CIMT was normally distributed with a median of 0.75 mm. The distribution of the 7 CVHI components, including health behaviors and health factors, by CIMT dichotomous categories (greater or less than the median of 0.75) is presented in Table 3. Using mixed‐model regression adapted for twin studies, all health factors (blood pressure, fasting glucose, and total cholesterol, fasting glucose) were significantly associated with CIMT categories with a higher proportion of subjects in the poor category of each component showing high versus low CIMT. Among the health behaviors, only BMI was significantly different comparing high and low CIMT categories (P=0.006). When all components of the CVHI were included together in the same model, blood pressure, fasting glucose, and BMI were independently associated with CIMT.
Table 3.
Distribution of CVHI Components and the Total CVHI by CIMT Categories in the Emory Twin Study
| CVHI Components | Categories | Low CIMT ≤0.75, n (%) | High CIMT >0.75, n (%) | P Value | CIMT Continuous (Coefficient in mm) | P Value |
|---|---|---|---|---|---|---|
| Blood pressure | Ideal | 51 (19.5) | 32 (13.9) | 0.04 | −0.02 | 0.008 |
| Intermediate | 133 (50.9) | 113 (49.3) | ||||
| Poor | 77 (29.5) | 84 (36.7) | ||||
| Fasting glucose | Ideal | 145 (55.6) | 104 (45.4) | 0.01 | −0.02 | 0.02 |
| Intermediate | 102 (39.1) | 103 (44.9) | ||||
| Poor | 14 (5.4) | 22 (9.6)) | ||||
| Total cholesterol | Ideal | 124 (47.5) | 93 (40.6) | 0.05 | −0.003 | 0.62 |
| Intermediate | 119 (45.6) | 111 (48.5) | ||||
| Poor | 18 (6.9) | 25 (10.9) | ||||
| Body mass index | Ideal | 48 (18.4) | 26 (11.3) | 0.006 | −0.024 | 0.007 |
| Intermediate | 125 (47.9) | 92 (40.2) | ||||
| Poor | 88 (33.7) | 111 (48.5) | ||||
| Physical activity | Ideal | 57 (25.7) | 50 (26.3) | 0.72 | 0.003 | 0.72 |
| Intermediate | 135 (60.8) | 109 (57.4) | ||||
| Poor | 30 (13.5) | 31 (16.3) | ||||
| Healthy diet | Ideal | 47 (19.1) | 44 (20.4) | 0.42 | 0.006 | 0.39 |
| Intermediate | 127 (51.6) | 117 (54.1) | ||||
| Poor | 72 (29.3) | 55 (25.5) | ||||
| Smoking | Ideal | 202 (77.4) | 165 (72.0) | 0.22 | −0.01 | 0.06 |
| Intermediate | 1 (1.0) | 2 (1.3) | ||||
| Poor | 59 (22.6) | 61 (26.6) | ||||
| CVHI categories | Optimum | 60 (23.0) | 29 (12.6) | 0.004 | −0.03 | <0.001 |
| Average | 193 (73.9) | 182 (79.5) | ||||
| Inadequate | 8 (3.1) | 18 (7.8) |
CIMT indicates carotid intima‐media thickness; CVHI, cardiovascular health index.
For the overall CVHI, more than twice the number of participants in the inadequate CVHI category had high versus low CIMT (7.8% versus 3.1%). Conversely, fewer participants in the optimum CVHI category had high versus low CIMT (12.6% versus 23.0%), P=0.004. When CIMT was analyzed as a continuous variable, results were similar (Table 3). For a change in CVHI category, from inadequate to average or average to optimum, CIMT decreased by 0.03 mm (P≤0.001). When the CVHI was treated as a continuous score, with a higher score indicative of better cardiovascular health, the score was negatively correlated with CIMT (Spearman r=−0.22, P<0.001) (Figure 2). Also, as the number of ideal health factors and health behaviors in participants increased, CIMT declined in a graded manner (Figure 3).
Figure 2.
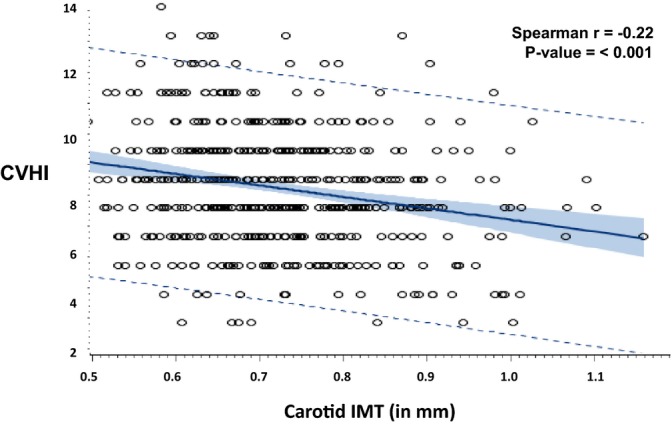
Correlation between cardiovascular health index (CVHI) and carotid intima‐media thickness (CIMT).
Figure 3.
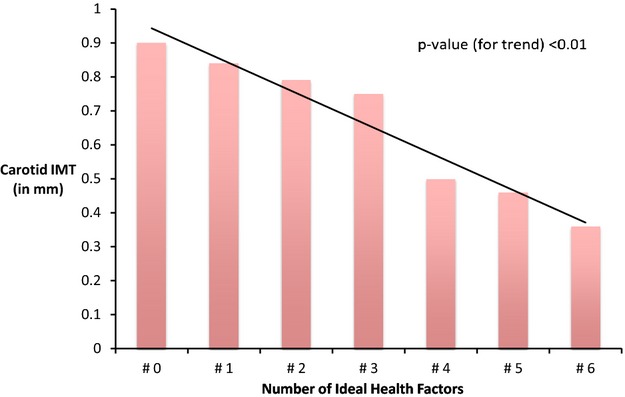
Bar plot of mean carotid intima‐media thickness (CIMT) according to number of ideal health factors.
In the unadjusted model, for every 5‐unit increase in overall CVHI score (indicating better cardiovascular health category), CIMT decreased by 0.045 mm (P<0.001). This association was mildly diminished after adjusting for age, college education, employment, and further adjusting for depression (P = 0.001). Cardiovascular health categories were also similarly associated with CIMT in the crude and adjusted models (Table 4).
Table 4.
Unadjusted and Adjusted Association Between CIMT and Overall CVHI and Cardiovascular Health Categories (n=490)
| Models | Overall CVHI | Cardiovascular Health Category, Mean CIMT in mm (95% CI) | ||||
|---|---|---|---|---|---|---|
| Coefficient (Mean CIMT Difference in mm Per 5 Unit Change in Score) | P Value | Optimum (CVHI=10 to 14) | Average (CVHI=5 to 9) | Inadequate (CVHI=0 to 4) | P Value | |
| Model‐1 (unadjusted) | −0.045 | <0.001 | 0.73 (0.71, 0.75) | 0.75 (0.74, 0.77) | 0.83 (0.78, 0.87) | <0.001 |
| Model‐2 (adjusted for age, college education, employment) | −0.015 | 0.001 | 0.72 (0.69, 0.75) | 0.74 (0.73, 0.76) | 0.81 (0.79, 0.87) | <0.001 |
| Model‐3 (further adjusted for, depression, medications*) | −0.05 | 0.001 | 0.69 (0.64, 0.74) | 0.71 (0.70, 0.77) | 0.83 (0.77, 0.89) | <0.007 |
CIMT indicates carotid intima‐media thickness; CVHI, cardiovascular health index.
Medications include beta‐blockers, aspirin, angiotensin‐converting enzyme inhibitors, diabetes medication, and statins.
Our final analyses focused on twin pairs who were discordant for CVHI, that is, where one member of the twin pair had a higher CVHI score than the other. The CVHI score was continuous for this analysis and the results were stratified by zygosity. There were a total of 197 discordant twin pairs, 76 were DZ discordant and 121 were MZ discordant. Among MZ twins discordant for CVHI, for each 5‐unit difference in CVHI score between the 2 twins (inadequate to average or average to optimum category), CIMT differed by 0.05 mm (P<0.001). However, among DZ twins discordant for CVHI, CIMT was not significantly associated with the CVHI (P=0.18). Further adjustment for potential confounders did not alter the results (Table 5). The coefficients tended to be larger in the MZ than the DZ pairs, but the P value for the interaction with zygosity did not reach statistical significance (P=0.06 in the final model).
Table 5.
Unadjusted and Adjusted Within Pair Association of CVHI (Per 5 Unit Change in Overall Score) With CIMT (in mm)
| Models | MZ+DZ Pairs (n=394) | MZ Pairs (n=242) | DZ Pairs (n=152) | |||
|---|---|---|---|---|---|---|
| Coefficient (Mean CIMT Difference in mm Per 5 Unit Change in Score) | P Value | Coefficient (Mean CIMT Difference in mm Per 5 Unit Change in Score) | P Value | Coefficient (Mean CIMT Difference in mm Per 5 Unit Change in Score | P Value | |
| Model‐1 (unadjusted) | −0.045 | <0.001 | −0.05 | <0.001 | −0.03 | 0.18 |
| Model‐2 (adjusted for age, college education, employment) | −0.04 | <0.001 | −0.05 | <0.001 | −0.025 | 0.26 |
| Model‐3 (further adjusted for depression, medications*) | −0.04 | <0.001 | −0.05 | <0.001 | −0.04 | 0.11 |
CIMT indicates carotid intima‐media thickness; CVHI, cardiovascular health index; DZ, dizygotic; MZ, monozygotic.
Medications include beta‐blockers, aspirin, angiotensin‐converting enzyme inhibitors, diabetes medication, and statins.
Discussion
CIMT is an important preclinical marker for CVD and predicts future clinical cardiovascular end points including coronary heart disease and stroke.14 Our study in a twin sample showed that the cardiovascular health index, a new metric developed by the AHA, is inversely associated with CIMT and this association is independent of shared genetic and familial factors. We also found that this association is stronger for CVHI health factors (blood pressure, fasting glucose, and total cholesterol) compared with CVHI behavioral factors (healthy diet, physical activity, and smoking), except BMI, which was highly associated with CIMT. Furthermore, the association of CVHI with CIMT was not substantially diminished when examined within pairs, suggesting that familial factors do not confound this association. Importantly, the association remained strong within MZ pairs, who are also matched for genetic factors. These results suggest a possible causal relationship between health factors and CIMT. To our knowledge, ours is the first study to demonstrate a link between the CVHI, a new public health metric and CIMT, a marker of “vascular health.” Our study confirms the value of measuring the CVHI and suggests that it can be applied to the prevention of preclinical atherosclerosis, as measured by CIMT. The combination of health factors and unhealthy behaviors is a strong predictor of plaque burden. The use of these combined factors could play a role in CVD prevention in the preclinical phase.
CVHI was defined and created by AHA as part of a national effort to improve cardiovascular health of all Americans by 20% by 2020.7 The prevalence of ideal cardiovascular health is very low in the United States as measured in several studies.36–40 The distribution of ideal factors in our sample of middle‐aged male Vietnam era veterans was comparable to what was reported in National Health and Nutrition Examination Survey, but health factors and behaviors were less favorable in the Emory Twin Study than the United States' general population. Veterans are more likely to have health insurance as compared to nonveterans but they have a more negative risk factor profile.41 Several studies using nationally representative samples have found that veterans are more likely than civilians to participate in recommended preventive care but may not always have better health outcomes.42–43
Our study shows that CIMT was inversely associated with optimum CVHI and the association was independent of shared genetic and familial factors. Furthermore, as the number of ideal health factors increased, the CIMT showed a gradient decline. Poorer levels of CVHI have shown to be associated with several chronic diseases and mortality.36,39,44–46 Thus, measurement of CIMT in asymptomatic people with less than optimum health could potentially inform more aggressive management approaches and help in the global prevention of many diseases. CIMT has been used as a quantitative index for evaluating the progression of atherosclerosis and as a surrogate endpoint in clinical trials. For an absolute CIMT difference of 0.1 mm, the future risk of a coronary event increases by 10% to 15%, and the stroke risk increases by 13% to 18%.14 Epidemiological studies have shown associations of CIMT with several CVD risk factors including smoking, BMI, blood pressure, and high blood cholesterol.15–17,15–48 However, there are limited data on the effect of diet and physical activity on CIMT.49–50 Our study shows that the overall association of CVHI and CIMT was primarily driven by health factors such as blood pressure, fasting glucose, and total cholesterol instead of health behaviors. A 0.01 mm increase in CIMT corresponds to a 1‐year CIMT progression associated with vascular aging.51–52 This difference in our analysis was roughly associated with 1 point difference in CVHI. Thus, each point increase in CVHI could potentially decrease vascular aging by one additional year.
Previous studies have indicated that genetic factors have a substantial influence on the variation of CIMT.53–54 Genetic factors and other familial factors also influence health behaviors and other cardiovascular risk factors.18 In our study, the association of CIMT and CVHI was stronger in MZ twins than DZ twins, indicating that the association with CVHI is unlikely to be confounded by genetic factors that might be shared between CIMT and CVHI components. In addition to genetic factors, our co‐twin design allowed us to control for many other familial risk factors, including socioeconomic status, maternal factors, and early familial environment.55
Our study has notable strengths. The co‐twin study design is very useful in strengthening causal inference in observational studies.55 Due to matching of genetic background, parental factors, and upbringing environment, in addition to demographic factors, MZ twins discordant for exposure can be considered as counterfactuals when examining the association of a given exposure with an outcome. Numerous unmeasured potential confounding factors are removed in comparisons within twin pairs. Thus, in our analysis, any difference in mean CIMT between co‐twins is more likely to be truly attributable to differences in CVHI scores between the twins than to unaccounted factors.
Our study also had the advantage of having standardized measurements of several cardiovascular risk factors and health behaviors, which we were able to use for ascertainment of the CVHI score. There are some limitations to our study. The sample is restricted to middle‐aged, male Vietnam‐era veterans, and our results may not be generalizable to women or younger subjects. We controlled for many known lifestyle factors and compared twins raised in the same family; however, despite the strengths of the co‐twin design, unmeasured confounding still cannot be completely ruled out.
In summary, our study shows that the CVHI is correlated with CIMT, which is an inexpensive, safe and noninvasive measure of vascular health. The association of CVHI and CIMT is driven by health factors (blood pressure, fasting glucose, and total cholesterol) and is independent of shared genes and early family environment. Understanding the CVHI and CIMT relationship may enhance prevention and intervention efforts to assist in more effective ways to target people with less than optimum levels of cardiovascular health.
Sources of Funding
This study was supported by K24HL077506, R01 HL68630, and R01 AG026255 to Dr Vaccarino, and by K24 MH076955, R01 MH056120, and R01 HL088726 to Dr Bremner from the National Institutes of Health; by the Emory University General Clinical Research Center MO1‐RR00039 and by grant 0245115N from the American Heart Association. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University. Dr Eufinger is supported on F31 HL107080.
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MBAmerican Heart Association Statistics, Committee, & Stroke Statistics, Subcommittee Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013; 127:143-152 [DOI] [PubMed] [Google Scholar]
- 2.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects—atherosclerosis risk in communities study. Arch Intern Med. 2007; 167:573-579 [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk‐factor profile and long‐term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle‐aged men and women. JAMA. 1999; 282:2012-2018 [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Sullivan LM, Wilson PW, Sempos CT, Sundstrom J, Kannel WB, Levy D, D'Agostino RB. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Intern Med. 2005; 142:393-402 [DOI] [PubMed] [Google Scholar]
- 5.Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd‐Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long‐term risk of cardiovascular and all‐cause mortality. JAMA. 2004; 292:1588-1592 [DOI] [PubMed] [Google Scholar]
- 6.Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121:948-954 [DOI] [PubMed] [Google Scholar]
- 7.Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010; 121:586-613 [DOI] [PubMed] [Google Scholar]
- 8.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B‐mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991; 134:250-256 [DOI] [PubMed] [Google Scholar]
- 9.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997; 146:483-494 [DOI] [PubMed] [Google Scholar]
- 10.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, Szklo M, Howard G, Evans GW. Risk factors for progression of common carotid atherosclerosis: the atherosclerosis risk in communities study, 1987–1998. Am J Epidemiol. 2002; 155:38-47 [DOI] [PubMed] [Google Scholar]
- 11.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999; 340:14-22 [DOI] [PubMed] [Google Scholar]
- 12.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam study. Circulation. 1997; 96:1432-1437 [DOI] [PubMed] [Google Scholar]
- 13.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2000; 151:478-487 [DOI] [PubMed] [Google Scholar]
- 14.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007; 115:459-467 [DOI] [PubMed] [Google Scholar]
- 15.Allan PL, Mowbray PI, Lee AJ, Fowkes FG. Relationship between carotid intima‐media thickness and symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke. 1997; 28:348-353 [DOI] [PubMed] [Google Scholar]
- 16.Prati P, Vanuzzo D, Casaroli M, Di Chiara A, De Biasi F, Feruglio GA, Touboul PJ. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992; 23:1705-1711 [DOI] [PubMed] [Google Scholar]
- 17.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999; 30:841-850 [DOI] [PubMed] [Google Scholar]
- 18.Lieb W, Vasan RS. Genetics of coronary artery disease. Circulation. 2013; 128:1131-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell CJ, Nabel EG. Cardiovascular genomics, personalized medicine, and the National Heart, Lung, and Blood Institute: Part I. The beginning of an era. Circ Cardiovasc Genet. 2008; 1:51-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touboul PJ, Labreuche J, Vicaut E, Amarenco P. Carotid intima‐media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005; 36:1741-1745 [DOI] [PubMed] [Google Scholar]
- 21.Baldassarre D, Amato M, Bondioli A, Sirtori CR, Tremoli E. Carotid artery intima‐media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke. 2000; 31:2426-2430 [DOI] [PubMed] [Google Scholar]
- 22.Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima‐media thickness in healthy young adults: The atherosclerosis risk in young adults (arya) study. Arch Intern Med. 2003; 163:1787-1792 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002; 5:476-481 [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Cheema FA, Reddy U, Bremner JD, Su S, Goldberg J, Snieder H, Vaccarino V. Heritability of flow‐mediated dilation: a Twin Study. J Thromb Haemost. 2007; 5:2386-2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, Goldberg J, Raggi P, Quyyumi AA, Bremner JD. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J Am Coll Cardiol. 2011; 57:1271-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AJ, Su S, Veledar E, Bremner JD, Goldstein FC, Lampert R, Goldberg J, Vaccarino V. Is heart rate variability related to memory performance in middle‐aged men? Psychosom Med. 2011; 73:475-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Beck Depression Inventory, 2nd edition (BDI‐II). 1996San Antonio, TX: Psychological Corporation [Google Scholar]
- 28.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck Depression Inventory‐II with adolescent psychiatric inpatients. Psychol Assess. 2004; 16:120-132 [DOI] [PubMed] [Google Scholar]
- 29.Pols MA, Peeters PH, Bueno‐De‐Mesquita HB, Ocke MC, Wentink CA, Kemper HC, Collette HJ. Validity and repeatability of a modified baecke questionnaire on physical activity. Int J Epidemiol. 1995; 24:381-388 [DOI] [PubMed] [Google Scholar]
- 30.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: A scientific statement from the american heart association. Hypertension. 2006; 47:296-308 [DOI] [PubMed] [Google Scholar]
- 31.Sacks FM, Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A dietary approach to prevent hypertension: A review of the dietary approaches to stop hypertension (dash) study. CClin Cardiol. 1999; 22:III6-10 [DOI] [PubMed] [Google Scholar]
- 32.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a dash‐style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008; 168:713-720 [DOI] [PubMed] [Google Scholar]
- 33.Salonen JT, Salonen R. Ultrasound B‐mode imaging in observational studies of atherosclerotic progression. Circulation. 1993; 87:II56-II65 [PubMed] [Google Scholar]
- 34.Coll B, Feinstein SB. Carotid intima‐media thickness measurements: techniques and clinical relevance. Curr Atheroscler Rep. 2008; 10:444-450 [DOI] [PubMed] [Google Scholar]
- 35.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005; 34:1089-1099 [DOI] [PubMed] [Google Scholar]
- 36.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57:1690-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012; 125:45-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan study. Circulation. 2012; 125:2975-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013; 44:1909-1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011; 123:850-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25‐64: United states, 2007–2010. NCHS Data Brief. 2012:1-8 [PubMed] [Google Scholar]
- 42.Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am J Prev Med. 2012; 43:483-489 [DOI] [PubMed] [Google Scholar]
- 43.Lehavot K, Hoerster KD, Nelson KM, Jakupcak M, Simpson TL. Health indicators for military, veteran, and civilian women. Am J Prev Med. 2012; 42:473-480 [DOI] [PubMed] [Google Scholar]
- 44.Muntner P, Judd SE, Gao L, Gutierrez OM, Rizk DV, McClellan W, Cushman M, Warnock DG. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol. 2013; 24:1159-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the atherosclerosis risk in communities study. Circulation. 2013; 127:1270-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the american heart association's definition in the reasons for geographic and racial differences in stroke (regards) study. PloS One. 2012; 7:e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O'Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the cardiovascular health study. JAMA. 1992; 268:1287-1291 [PubMed] [Google Scholar]
- 48.Gentile M, Iannuzzi A, Iannuzzo G, Covetti G, Panico S, Mattiello A, De Michele M, Rubba P. Relation of body mass index with carotid intima‐media thickness and diameter is independent of metabolic syndrome in postmenopausal mediterranean women. Menopause. 2012; 19:1104-1108 [DOI] [PubMed] [Google Scholar]
- 49.Bertoni AG, Whitt‐Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the multi‐ethnic study of atherosclerosis. Am J Epidemiol. 2009; 169:444-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity‐related vascular dysfunction in children. Circulation. 2004; 109:1981-1986 [DOI] [PubMed] [Google Scholar]
- 51.Cheng KS, Mikhailidis DP, Hamilton G, Seifalian AM. A review of the carotid and femoral intima‐media thickness as an indicator of the presence of peripheral vascular disease and cardiovascular risk factors. Cardiovasc Res. 2002; 54:528-538 [DOI] [PubMed] [Google Scholar]
- 52.Stein JH. Carotid intima‐media thickness and vascular age: you are only as old as your arteries look. J Am Soc Echocardiogr. 2004; 17:686-689 [DOI] [PubMed] [Google Scholar]
- 53.Juo SH, Lin HF, Rundek T, Sabala EA, Boden‐Albala B, Park N, Lan MY, Sacco RL. Genetic and environmental contributions to carotid intima‐media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke. 2004; 35:2243-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J, Cheema FA, Bremner JD, Goldberg J, Su S, Snieder H, Maisano C, Jones L, Javed F, Murrah N, Le NA, Vaccarino V. Heritability of carotid intima‐media thickness: a Twin Study. Atherosclerosis. 2008; 197:814-820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGue M, Osler M, Christensen K. Causal inference and observational research: the utility of twins. Perspect Psychol Sci. 2010; 5:546-556 [DOI] [PMC free article] [PubMed] [Google Scholar]


