Abstract
Objective:
This study was conducted to determine the relationship of frontal lobe cortical thickness and basal ganglia volumes to measures of cognition in adults with sickle cell anemia (SCA).
Methods:
Participants included 120 adults with SCA with no history of neurologic dysfunction and 33 healthy controls (HCs). Participants were enrolled at 12 medical center sites, and raters were blinded to diagnostic group. We hypothesized that individuals with SCA would exhibit reductions in frontal lobe cortex thickness and reduced basal ganglia and thalamus volumes compared with HCs and that these structural brain abnormalities would be associated with measures of cognitive functioning (Wechsler Adult Intelligence Scale, 3rd edition).
Results:
After adjusting for age, sex, education level, and intracranial volume, participants with SCA exhibited thinner frontal lobe cortex (t = −2.99, p = 0.003) and reduced basal ganglia and thalamus volumes compared with HCs (t = −3.95, p < 0.001). Reduced volume of the basal ganglia and thalamus was significantly associated with lower Performance IQ (model estimate = 3.75, p = 0.004) as well as lower Perceptual Organization (model estimate = 1.44, p = 0.007) and Working Memory scores (model estimate = 1.37, p = 0.015). Frontal lobe cortex thickness was not significantly associated with any cognitive measures.
Conclusions:
Our findings suggest that basal ganglia and thalamus abnormalities may represent a particularly salient contributor to cognitive dysfunction in adults with SCA.
Neurocognitive deficits among children with sickle cell anemia (SCA) have been reported in a variety of domains,1–6 but deficiencies in intellectual function (IQ) have been the most consistently documented.3,6–10 Cognitive dysfunction in SCA appears to worsen with age,7,11 and individuals with SCA are now living longer.12 Unfortunately, few studies have evaluated cognitive functioning or associated structural brain abnormalities in adults with SCA. One previous study by our research group demonstrated that neurologically asymptomatic adults with SCA exhibited prominent weaknesses on measures of IQ, but no link to structural brain abnormalities was documented.13
In children with SCA, the association between cerebrovascular lesions and IQ is well established.5,6,8,9,14,15 However, because a significant proportion of both children and adults with SCA do not have cerebrovascular lesions,5,13,16 additional CNS factors likely contribute to cognitive dysfunction. Frontal lobe cortical atrophy17–21 and gray matter growth delays in the basal ganglia and thalamus reported in children and adolescents with SCA22–24 may represent factors contributing to poorer intellectual function in adults with SCA. This study was conducted to evaluate MRI characteristics of neurologically asymptomatic adults with SCA to determine the relationship of these characteristics with IQ. We hypothesized that participants with SCA would exhibit reductions in frontal lobe cortex thickness and reduced subcortical gray matter (basal ganglia and thalamus) volume, which would be significantly associated with measures of IQ.
METHODS
Participants.
Participants were enrolled from 12 medical center sites that were part of the Comprehensive Sickle Cell Centers program in the United States from December 2004 to May 2008. Adults with SCA were excluded if they had a history of prior head injury resulting in neurologic symptoms or medical visit, stroke, abnormal neurologic examination or neuroimaging findings; chronic end-organ disease; or evidence of depression at baseline screening. Neurologically asymptomatic adults with SCA (n = 160) and healthy controls (HCs) (n = 52) were enrolled and stratified using a 3:1 ratio. Controls were matched for race, age, sex, and education. Eligibility criteria, participant disposition, and the participating centers were previously described.13 Of the eligible participants, 146 adults with SCA and 47 HCs completed the cognitive assessment, and 141 adults with SCA and 44 HCs had MRI scans. Of these, 120 participants with SCA and 33 HCs had scans of sufficient quality to complete volumetric and cortical thickness analyses. None of the participants were excluded from participation on the basis of abnormal MRI results.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at all sites approved all aspects of this study. Written informed consent was provided by all participants.
MRI evaluation.
The University of California, San Francisco Center for Imaging of Neurodegenerative Diseases was the MRI reading center.
Imaging acquisition.
All MRI scans were performed on 1.5-tesla scanners on various platforms across the 12 sites. The imaging sequence used for volumetric analyses was a 3-dimensional T1-weighted image magnetization-prepared rapid-acquisition gradient echo (MP-RAGE) or spoiled gradient recalled echo. The nominal parameters of the MP-RAGE were as follows: coronal plane, repetition time (TR)/echo time (TE)/T1 = 10/4/300 milliseconds, flip angle 15°, 256 × 256 in-plane matrix, and 1.5-mm slice thickness. The nominal parameters of the spoiled gradient recalled echo were comparable to that of the MP-RAGE—coronal plane, TR/TE/T1 = 9/4/300 milliseconds, flip angle 15°, 256 × 256 in-plane matrix, and 1.5-mm slice thickness—but varied slightly across sites within an acceptable range. TR range was 9–11 milliseconds, TE range was 4–5 milliseconds, and flip angle was 15° or 20°. To ensure between-site consistency, scanner qualification and monitoring were performed at the Center for Imaging of Neurodegenerative Diseases using the American College of Radiology phantom and its qualification routine.25 Once scanners were harmonized using phantoms, each site then performed a test-retest scan on a volunteer to confirm that sequences were stabilized in repeat human brain scans.
Image processing.
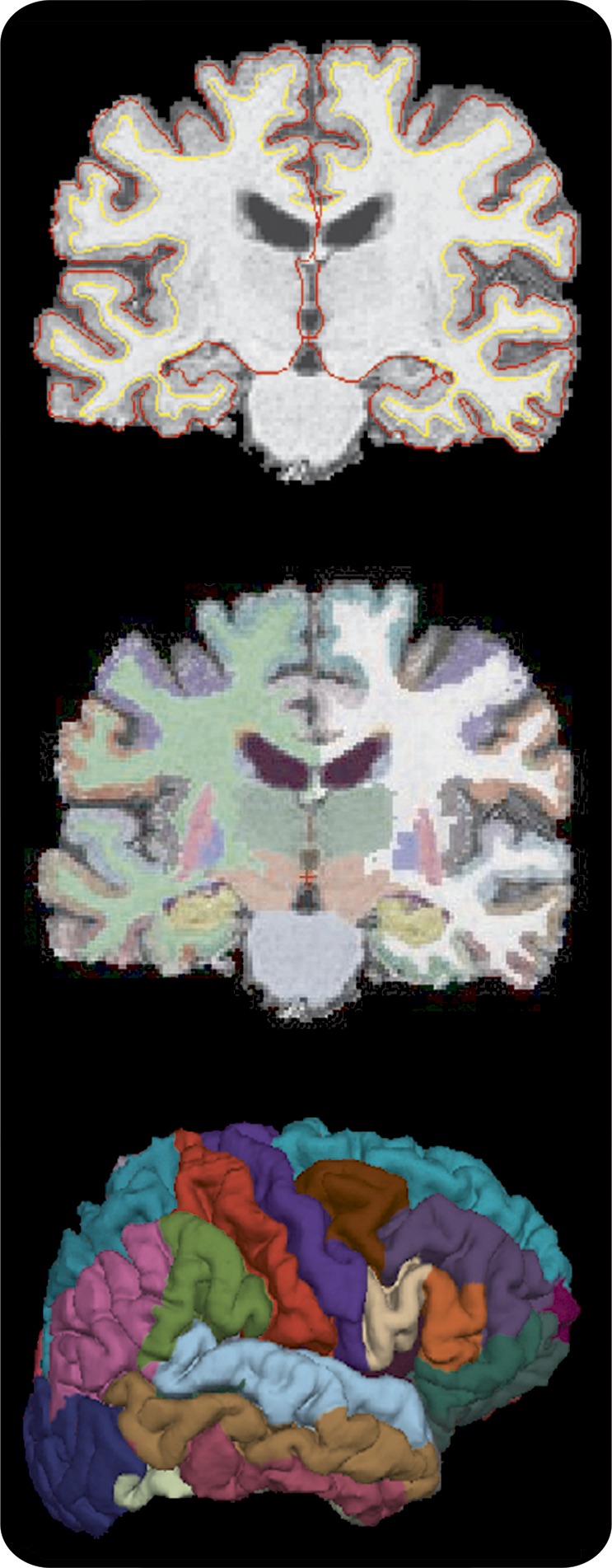
The image processing was performed using FreeSurfer version 4.5 (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer is a fully automated image-processing suite that provides cortical thickness and volume measurements in 116 total cortical and subcortical regions of the brain (figure). The technical details of these procedures are described elsewhere.26–28 Primary regions of interest for this study included volumetric measurement of the basal ganglia and thalamus, thickness of the frontal lobe cortex, volume of white matter hypointensities, and the presence of lacunes. Basal ganglia volumes included the putamen, caudate, and pallidum. A basal ganglia + thalamus total volume was calculated by summing the volume of each of these structures and averaging across the 2 hemispheres. Frontal lobe cortical thickness was obtained by summing average thickness measurements for each subregion of the frontal lobe and averaging thickness measurements across the 2 hemispheres. Thickness measurements of temporal, occipital, and parietal lobes were also calculated for secondary analyses in the same manner. Total intracranial volume (ICV) was used in regression models to account for variability in head size. White matter abnormalities were evaluated 2 ways: (1) volumes of white matter hypointensities were obtained utilizing the T1 MRI using FreeSurfer, and (2) the presence and severity of lacunes was rated by independent and blinded raters using standardized criteria.
Figure. FreeSurfer parcellation of the brain.
Neurocognitive assessment.
Neurocognitive evaluations at each site were conducted by neuropsychologists trained in the administration and scoring of the tests.13 All neuropsychological data were reviewed and validated by the coordinating center neuropsychologist for quality purposes. The primary cognitive outcomes were the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III)29 Performance IQ (PIQ), Processing Speed Index (PSI), Perceptual Organization Index (POI), Working Memory Index (WMI), and Full Scale IQ (FSIQ). Secondary outcome variables included the WAIS-III Verbal IQ and Verbal Comprehension Index. All cognitive scores were adjusted for age at the time of testing.
Statistical analysis.
The 2 groups were compared on demographic and baseline measures using Fisher exact tests for categorical variables (education ≤12 years vs >12 years, sex) and Mann-Whitney tests for continuous variables. To compare patients and controls on the primary cognitive outcomes for each MRI region, linear regression models were fit to the data. When comparing groups, each regression model was adjusted for age, sex, education, and ICV. An exploratory analysis of secondary MRI regions was done, and the resulting p values were adjusted using a false discovery rate correction30 after controlling for the effect of ICV, age, sex, and education. Group performance was evaluated using a linear mixed-effects model on measures of cognitive functioning. These models included age, sex, education, and a diagnosis by cognitive subscale interaction. They were fit using a random intercept and a compound symmetry correlation structure. Subsequently, linear regression models were fit to evaluate the relationship between cognitive performance variables differentiating the 2 groups (PIQ, PSI, POI, WMI, FSIQ) and MRI regions of interest, while adjusting for age, sex, education, and hemoglobin within the SCA group. Volumes and thicknesses were centered on their means and scaled by their SDs. Additional analyses were performed, excluding subjects with lacunes, to evaluate their effect on the results. Model fits were evaluated by an analysis of the residuals. Statistical analyses were generated using R version 2.12.2.
RESULTS
Demographic and clinical characteristics.
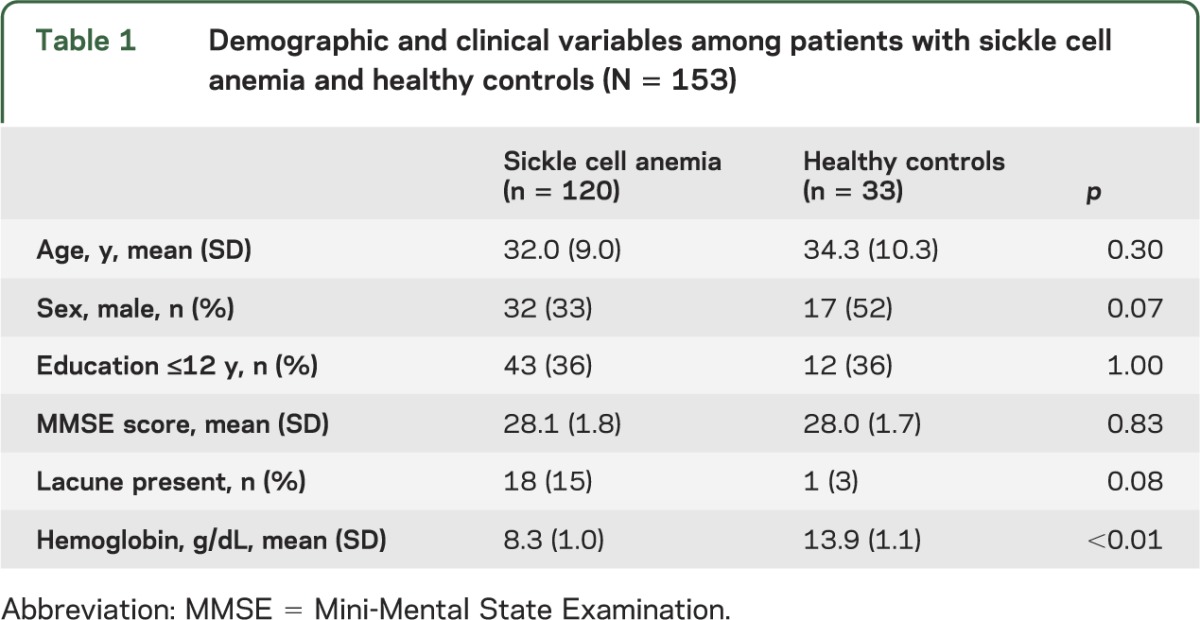
There were no significant differences between SCA and HC participants regarding age, education, sex, or Mini-Mental State Examination score (table 1). The mean age of the entire sample was 31.9 years and participants ranged in age from 19.8 to 55.8 years; 60% of participants were female, 65% completed 12 or more years of education, and the mean Mini-Mental State Examination score was 28.2 (SD = 1.7). Compared with controls, participants with SCA had lower mean hemoglobin values (8.3 g/dL [SD = 1.0] vs 13.8 g/dL [SD = 1.1]), consistent with previously published findings.13 Although participants with SCA had a higher incidence of visually rated lacunes than controls (15% vs 3%), this was not significantly different between the groups. Additional health history data are provided in table e-1 on the Neurology® Web site at Neurology.org.
Table 1.
Demographic and clinical variables among patients with sickle cell anemia and healthy controls (N = 153)
MRI results.
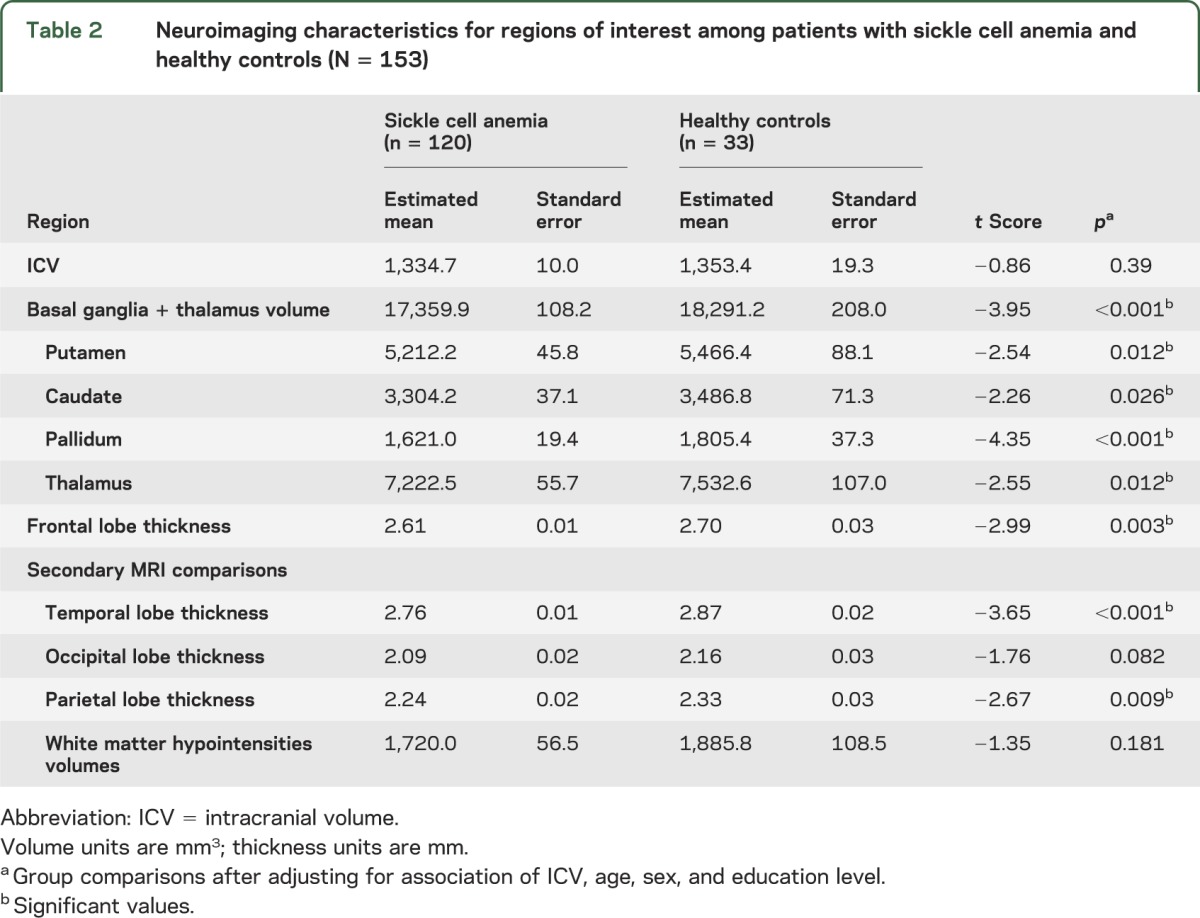
Comparison of the MRI regions of interest between participants with SCA and HCs is shown in table 2. After adjusting for age, sex, education level, and ICV, participants with SCA exhibited thinner cortex of the frontal lobe relative to HCs (t = −2.99, p = 0.003). Participants with SCA also demonstrated smaller basal ganglia and thalamus volumes compared with controls (adjusted mean 17,359.9 mm3 for participants with SCA vs 18,297.2 mm3 for controls, t = −3.95, p < 0.001), and these smaller volumes were evident in each of the subregions comprising the total basal ganglia and thalamus volume: caudate (t = −2.26, p = 0.026), pallidum (t = −4.35, p < 0.001), putamen (t = −2.54, p = 0.012), and thalamus (t = −2.55, p = 0.012). Age was significantly associated with brain structure, but the effect of age did not differ between groups and scanner site was not significantly associated with brain structure measurement. In secondary analyses, participants with SCA had smaller thickness of the temporal lobe (t = −3.65, p < 0.001) and parietal lobe (t = −2.68, p = 0.009) relative to HCs after adjusting for multiple comparisons. The 2 groups did not differ on occipital lobe thickness. Furthermore, the SCA and control groups did not differ significantly on presence of lacunes or volume of white matter hypointensities, and white matter hypointensity volumes also did not differ between groups after removing individuals with lacunes. Of note, in exploratory analyses, basal ganglia and thalamus volume was not associated with hemoglobin level (t = 1.42, p = 0.16).
Table 2.
Neuroimaging characteristics for regions of interest among patients with sickle cell anemia and healthy controls (N = 153)
Cognitive functioning.
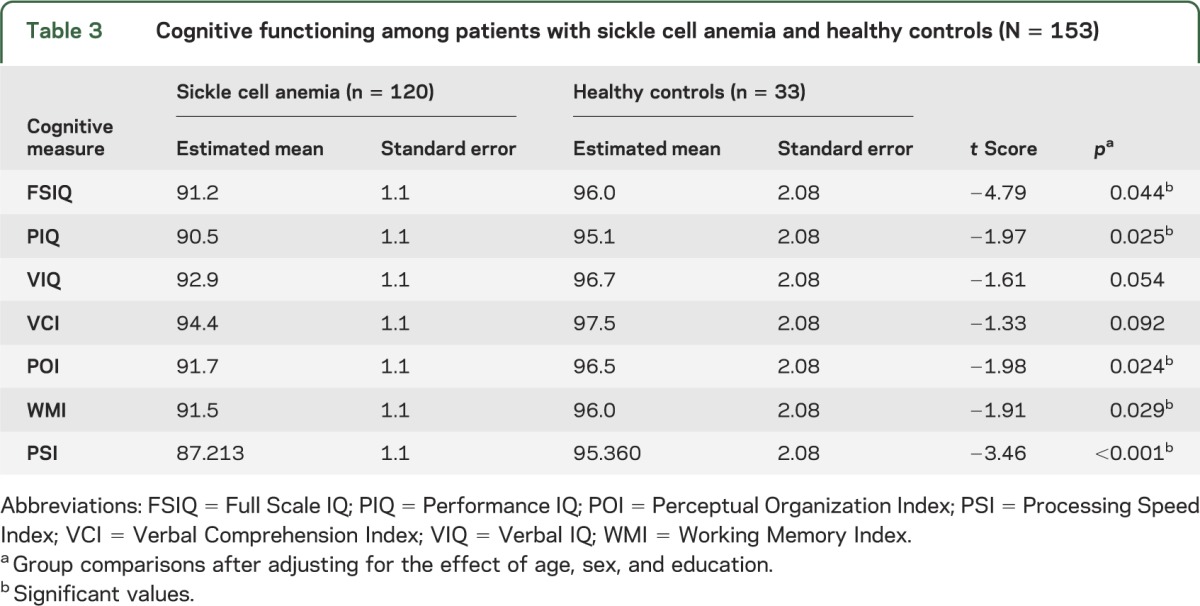
Consistent with previous findings,13 after controlling for age, sex, and education level, participants with SCA who had an MRI scan demonstrated poorer performance than controls on PIQ (t = −1.97, p = 0.03; table 3) as well as global intelligence (FSIQ; t = −4.79, p = 0.04), processing speed (PSI; t = −3.46, p < 0.001), perceptual organization (POI; t = −1.98, p = 0.02), and working memory (WMI; t = −1.91, p = 0.03). Performance on measures of Verbal IQ (t = −1.61, p = 0.054) and Verbal Comprehension Index (t = −1.33, p = 0.09) was not statistically different between groups. These same patterns were observed after removing individuals with lacunes from the sample. Of note, the effect of age on cognitive performance was not significantly different between groups.
Table 3.
Cognitive functioning among patients with sickle cell anemia and healthy controls (N = 153)
Association of MRI regions of interest and cognitive functioning in adults with SCA.
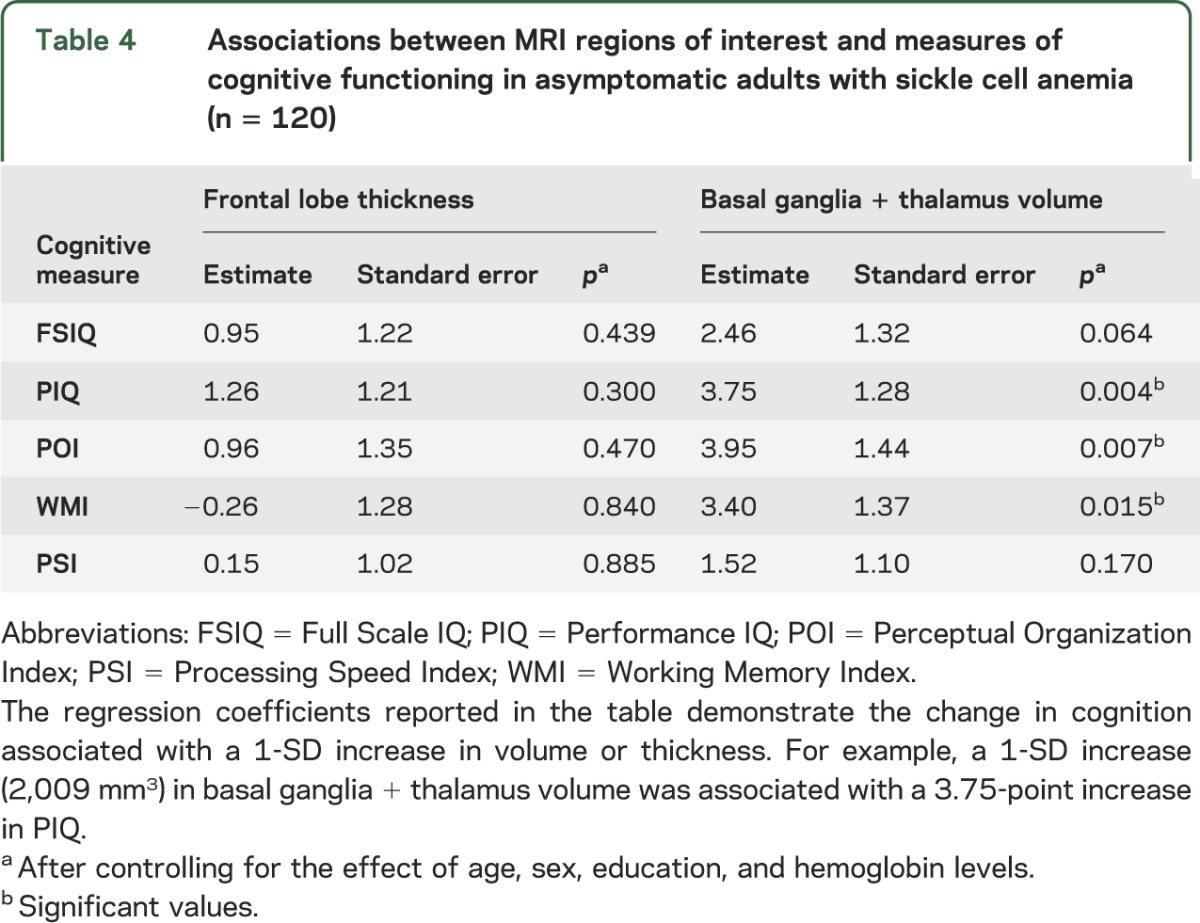
Results of the linear mixed-effects models evaluating the association of cortical thickness and basal ganglia and thalamus volumes with cognitive functioning are shown in table 4. After accounting for the effects of age, sex, education, and hemoglobin level, total volume of the basal ganglia and thalamus was significantly associated with PIQ (model estimate = 3.75, p = 0.004), POI (model estimate = 1.44, p = 0.007), and WMI (model estimate = 1.37, p = 0.015). Associations between cognitive function and hemoglobin levels were not significant. ICV was removed from the model because of multicollinearity with other variables. The volume of basal ganglia and thalamus was not significantly associated with FSIQ or PSI. Frontal lobe thickness was not significantly associated with any of the measures of cognitive performance after accounting for demographic and clinical characteristics.
Table 4.
Associations between MRI regions of interest and measures of cognitive functioning in asymptomatic adults with sickle cell anemia (n = 120)
Exploratory brain structure differences between SCA and control groups.
After testing our a priori hypotheses, we conducted exploratory analyses comparing brain structure differences between SCA and control participants. FreeSurfer was used to evaluate 34 regions for cortical thickness (averaged across hemispheres) and 3 subcortical gray matter volumes that were not included in our primary analyses (hippocampus, amygdala, nucleus accumbens). After correcting for multiple comparisons and controlling for ICV, age, sex, and education, 17 regions differentiated the 2 groups (table e-2). In the SCA group, statistically significant reductions were found in measures of frontal, parietal, and temporal lobe cortical thickness and volume of the amygdala.
DISCUSSION
Previous studies have suggested that frontal lobe abnormalities may be associated with IQ deficiencies in children with SCA22 and that metabolic abnormalities and gray matter growth delays occur in the basal ganglia and thalamus in children with SCA.17–21 However, structural brain abnormalities of cortical thickness or basal ganglia volumes in neurologically asymptomatic adults with SCA have not been reported. Therefore, our observation that reduced cortical thickness and reduced volumes of subcortical gray matter are evident in adults with SCA is important. Furthermore, although the link between cerebrovascular lesions and SCA is well established,31,32 we did not observe a greater burden of white matter abnormalities measured by white matter lesion volumes or visual ratings of the presence and severity of lacunes in participants with SCA relative to controls. As a result, we suspect that the observed abnormalities in cortical and subcortical gray matter structures are not primarily the result of cerebrovascular disease.
We first replicated our prior findings of cognitive dysfunction in adults with SCA, which was also consistent with numerous studies documenting cognitive performance deficiencies in children with SCA.3,6–10 We then replicated these results after removing all individuals with cerebrovascular lesions, indicating that cerebrovascular lesions are not the only contributors to deficient cognitive functioning in SCA,5,6,8,9,14,15 and we also found that the effect of age on cognition did not differ between participants with SCA and controls. Taken together, our results suggest that (1) cognitive dysfunction is evident in neurologically asymptomatic SCA, (2) age does not affect cognitive performance of individuals with SCA to a greater degree than controls, and (3) cognitive dysfunction cannot be adequately explained by cerebrovascular lesions. These findings underscore the potential contribution of other structural brain abnormalities to cognitive dysfunction in SCA.
Our most significant observation is the association of basal ganglia and thalamus volumes to measures of cognitive functioning (PIQ, POI, WMI) in adults with SCA accounting for the presence of lacunes. These findings represent the first published report of structural brain abnormalities, other than cerebrovascular lesions, to be associated with cognitive dysfunction in adults with SCA. Because a significant proportion of both children and adults with SCA do not have cerebrovascular lesions,5,13,16 our findings suggest that basal ganglia and thalamus abnormalities may represent a salient contributor to cognitive dysfunction in adults with SCA. This conceptualization is consistent with emerging data supporting the role of the basal ganglia in cognitive set shifting, planning, and visuospatial attention.33,34
Our hypothesis that cognitive dysfunction is associated with frontal lobe thickness was not supported. These findings are inconsistent with a recent study reporting that cortical atrophy, particularly in the frontal lobes, may be a particularly salient aspect of SCA contributing to IQ deficits in children with SCA22 and healthy children without SCA.35 Of note, it is possible that our use of an aggregate bilateral measure of frontal lobe thickness, rather than investigation of lateralized subregions of the frontal lobe in association with cognition,36 contributed to our results. Furthermore, although frontal lobe cortical thickness was not associated with cognition, our exploratory analyses found that there are other cortical thickness abnormalities evident in temporal and parietal regions in SCA. As such, the association of additional cortical abnormalities and lateralized cortical associations with cognition in adults with SCA represent important factors to be explored in future studies. Also, the impact of hemoglobin on cognitive function was not statistically significant after accounting for other clinical and demographic characteristics. Our current findings suggest that this relationship may not be as robust as was found in our previous report,13 and additional future studies will clarify this association.
The etiology of structural brain abnormalities and cognitive dysfunction in adult patients with SCA is likely complex. In addition to vasculopathy, adults with SCA experience cumulative morbidity as a result of multiorgan dysfunction secondary to inflammatory processes. In the brain, this may contribute to the patterns of regional brain atrophy and associated cognitive dysfunction we observed. Additionally, arthritis, sleep apnea, and chronic pain are also common manifestations of SCA that have been associated with changes in brain morphology, including the basal ganglia and thalamus, and cognitive dysfunction.37–40 As novel therapies addressing inflammation and hypoxia reperfusion injury in SCA are entering clinical trials, these interventions may alter the CNS pathology of this disease and associated cognitive dysfunction.
Our study had several limitations. First, our sample consisted of neurologically intact adult patients with SCA who sought treatment and agreed to participate in this study. They were not representative of the larger SCA patient population. Second, our a priori hypotheses were limited regarding brain regions that we compared between the 2 groups. Subsequent exploratory comparisons suggested additional regions of interest. Third, our study was cross-sectional and although subcortical gray matter volumes were associated with poorer performance on measures of cognitive function, we cannot conclude that this is a causal relationship.
Among neurologically intact adults with SCA, cortical thickness abnormalities and smaller subcortical gray matter volume in the basal ganglia and thalamus were prominent. White matter abnormalities were not evident in patients with SCA relative to controls. Structural brain abnormalities in the basal ganglia and thalamus were associated with cognitive dysfunction in patients with SCA but frontal lobe thickness and white matter abnormalities were not. Our results indicate that subcortical gray matter abnormalities may be an important and understudied contributor to cognitive dysfunction in patients with SCA. Clarification of these relationships may lead to improved intervention for cognitive dysfunction in neurologically intact adults with SCA.
Supplementary Material
GLOSSARY
- FSIQ
Full Scale IQ
- HC
healthy control
- ICV
intracranial volume
- MP-RAGE
magnetization-prepared rapid-acquisition gradient echo
- PIQ
Performance IQ
- POI
Perceptual Organization Index
- PSI
Processing Speed Index
- SCA
sickle cell anemia
- TE
echo time
- TR
repetition time
- WAIS-III
Wechsler Adult Intelligence Scale, 3rd edition
- WMI
Working Memory Index
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
R. Scott Mackin and Philip Insel: study design, data analysis, writing the manuscript, and editing/reviewing the manuscript. Diana Truran: study design, data collection, data analysis, writing the manuscript, and editing/reviewing the manuscript. Elliot P. Vichinsky: study design, data collection, implementing intervention/treatment, data analysis, writing the manuscript, and editing/reviewing the manuscript. Lynne D. Neumayr: study design, data analysis, writing the manuscript, and editing/reviewing the manuscript. F.D. Armstrong: study design, data collection, data analysis, writing the manuscript, and editing/reviewing the manuscript. Jeffrey I. Gold: study design, data collection/management, and editing/reviewing the manuscript. Karen Kesler: study design, data analysis, and editing/reviewing the manuscript. Joseph Brewer: data collection, data analysis, and editing/reviewing the manuscript. Michael W. Weiner: study design, data analysis, writing the manuscript, and editing/reviewing the manuscript.
STUDY FUNDING
Supported by the National Heart, Lung, and Blood Institute (U54HL070587) and the National Institute of Mental Health (K08MH081065).
DISCLOSURE
R. Mackin, P. Insel, D. Truran, E. Vichinsky, L. Neumayr, F. Armstrong, J. Gold, K. Kesler, and J. Brewer report no disclosures. M. Weiner served on the scientific advisory board for Lilly, Araclon and Institut Català de Neurociències Aplicades, Gulf War Veterans Illnesses Advisory Committee, VACO, Biogen Idec, Pfizer, and BOLT International, was a consultant for AstraZeneca, Araclon, Medivation/Pfizer, Ipsen, TauRX Therapeutics Ltd., Bayer Healthcare, Biogen Idec, ExonHit Therapeutics, Servier, Synarc, Pfizer, Janssen, Harvard University, and KLJ Associates, and was funded for travel by NeuroVigil, Inc., CHRU-Hospital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Català de Neurociències Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer's Disease, Pfizer, AD PD meeting, Paul Sabatier University, Novartis, Tohoku University, Fundacio ACE, and Travel eDreams, Inc. Dr. Weiner served on the editorial advisory board for Alzheimer's & Dementia and Magnetic Resonance Imaging, received honoraria from NeuroVigil, Inc., Institut Català de Neurociències Aplicades, PMDA/Japanese Ministry of Health, Labour and Welfare, Tohoku University, and Alzheimer's Drug Discovery Foundation, received commercial research support from Merck and Avid, received government research support from DOD and VA, holds stock options in Synarc and Elan, and worked with the following organizations contributing to the Foundation for NIH and thus to the NIA-funded Alzheimer's Disease Neuroimaging Initiative: Abbott, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Anonymous Foundation, AstraZeneca, Bayer Healthcare, BioClinica, Inc. (ADNI 2), Bristol-Myers Squibb, Cure Alzheimer's Fund, Eisai, Elan, Gene Network Sciences, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly & Company, Medpace, Merck, Novartis, Pfizer Inc., Roche, Schering-Plough, Synarc, and Wyeth. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Noll RB, Stith L, Gartstein MA, et al. Neuropsychological functioning of youths with sickle cell disease: comparison with non-chronically ill peers. J Pediatr Psychol 2001;26:69–78 [DOI] [PubMed] [Google Scholar]
- 2.Scantlebury N, Mabbott D, Janzen L, et al. White matter integrity and core cognitive function in children diagnosed with sickle cell disease. J Pediatr Hematol Oncol 2011;33:163–171 [DOI] [PubMed] [Google Scholar]
- 3.Schatz J, Finke R, Roberts CW. Interactions of biomedical and environmental risk factors for cognitive development: a preliminary study of sickle cell disease. J Dev Behav Pediatr 2004;25:303–310 [DOI] [PubMed] [Google Scholar]
- 4.White DA, Moinuddin A, McKinstry RC, Noetzel M, Armstrong M, DeBaun M. Cognitive screening for silent cerebral infarction in children with sickle cell disease. J Pediatr Hematol Oncol 2006;28:166–169 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong FD, Thompson RJ, Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease: Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996;97(6 pt 1):864–870 [PubMed] [Google Scholar]
- 6.Watkins KE, Hewes DK, Connelly A, et al. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev Med Child Neurol 1998;40:536–543 [DOI] [PubMed] [Google Scholar]
- 7.Steen RG, Fineberg-Buchner C, Hankins G, Weiss L, Prifitera A, Mulhern RK. Cognitive deficits in children with sickle cell disease. J Child Neurol 2005;20:102–107 [DOI] [PubMed] [Google Scholar]
- 8.Steen RG, Miles MA, Helton KJ, et al. Cognitive impairment in children with hemoglobin SS sickle cell disease: relationship to MR imaging findings and hematocrit. AJNR Am J Neuroradiol 2003;24:382–389 [PMC free article] [PubMed] [Google Scholar]
- 9.Schatz J, White DA, Moinuddin A, Armstrong M, DeBaun MR. Lesion burden and cognitive morbidity in children with sickle cell disease. J Child Neurol 2002;17:891–895 [PubMed] [Google Scholar]
- 10.Wasserman AL, Wilimas JA, Fairclough DL, Mulhern RK, Wang W. Subtle neuropsychological deficits in children with sickle cell disease. Am J Pediatr Hematol Oncol 1991;13:14–20 [DOI] [PubMed] [Google Scholar]
- 11.Ruffieux N, Njamnshi AK, Wonkam A, et al. Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol 2013;19:143–160 [DOI] [PubMed] [Google Scholar]
- 12.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–1644 [DOI] [PubMed] [Google Scholar]
- 13.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 2010;303:1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc 2006;12:24–33 [DOI] [PubMed] [Google Scholar]
- 15.Gold JI, Johnson CB, Treadwell MJ, Hans N, Vichinsky E. Detection and assessment of stroke in patients with sickle cell disease: neuropsychological functioning and magnetic resonance imaging. Pediatr Hematol Oncol 2008;25:409–421 [DOI] [PubMed] [Google Scholar]
- 16.Grueneich R, Ris MD, Ball W, et al. Relationship of structural magnetic resonance imaging, magnetic resonance perfusion, and other disease factors to neuropsychological outcome in sickle cell disease. J Pediatr Psychol 2004;29:83–92 [DOI] [PubMed] [Google Scholar]
- 17.Emam AT, Ali AM, Babikr MA. Magnetic resonance spectroscopy of the brain in children with sickle cell disease. Neurosciences 2009;14:364–367 [PubMed] [Google Scholar]
- 18.Steen RG, Ogg RJ. Abnormally high levels of brain N-acetylaspartate in children with sickle cell disease. AJNR Am J Neuroradiol 2005;26:463–468 [PMC free article] [PubMed] [Google Scholar]
- 19.Steen RG, Xiong X, Mulhern RK, Langston JW, Wang WC. Subtle brain abnormalities in children with sickle cell disease: relationship to blood hematocrit. Ann Neurol 1999;45:279–286 [DOI] [PubMed] [Google Scholar]
- 20.Steen RG, Reddick WE, Mulhern RK, et al. Quantitative MRI of the brain in children with sickle cell disease reveals abnormalities unseen by conventional MRI. J Magn Reson Imaging 1998;8:535–543 [DOI] [PubMed] [Google Scholar]
- 21.Steen RG, Langston JW, Ogg RJ, Xiong X, Ye Z, Wang WC. Diffuse T1 reduction in gray matter of sickle cell disease patients: evidence of selective vulnerability to damage? Magn Reson Imaging 1999;17:503–515 [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Pawlak MA, Flynn TB, Krejza J, Herskovits EH, Melhem ER. Brain morphometry and intelligence quotient measurements in children with sickle cell disease. J Dev Behav Pediatr 2009;30:509–517 [DOI] [PubMed] [Google Scholar]
- 23.Raznahan A, Higgins NP, Griffiths PD, Humphrey A, Yates JR, Bolton PF. Biological markers of intellectual disability in tuberous sclerosis. Psychol Med 2007;37:1293–1304 [DOI] [PubMed] [Google Scholar]
- 24.Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage 2005;25:320–327 [DOI] [PubMed] [Google Scholar]
- 25.ACR MRI Quality Control Manual. Reston, VA: American College of Radiology; 2001 [Google Scholar]
- 26.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22 [DOI] [PubMed] [Google Scholar]
- 28.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006;32(1):180–194 [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. San Antonio: Psychological Corporation; 1997 [Google Scholar]
- 30.Benjamini Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300 [Google Scholar]
- 31.Wang WC, Pavlakis SG, Helton KJ, et al. MRI abnormalities of the brain in one-year-old children with sickle cell anemia. Pediatr Blood Cancer 2008;51:643–646 [DOI] [PubMed] [Google Scholar]
- 32.Baldeweg T, Hogan AM, Saunders DE, et al. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann Neurol 2006;59:662–672 [DOI] [PubMed] [Google Scholar]
- 33.Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 2006;59:257–264 [DOI] [PubMed] [Google Scholar]
- 34.Fielding J, Georgiou-Karistianis N, White O. The role of the basal ganglia in the control of automatic visuospatial attention. J Int Neuropsychol Soc 2006;12:657–667 [DOI] [PubMed] [Google Scholar]
- 35.Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 2006;31:993–1003 [DOI] [PubMed] [Google Scholar]
- 36.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage 2008;41:675–681 [DOI] [PubMed] [Google Scholar]
- 37.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum 2010;62:2930–2940 [DOI] [PubMed] [Google Scholar]
- 38.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res 2009;18:36–48 [DOI] [PubMed] [Google Scholar]
- 39.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410–10415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med 2006;7:60–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


