Table 1.
Screening for the optimal base.a)
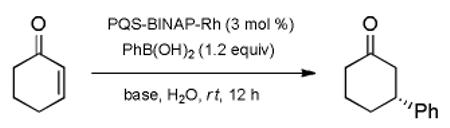
| entry | base | yieldb)[%] | eec)[%] |
|---|---|---|---|
| 1 | KOH | <5 | - |
| 2 | DIPEA | 61 | 98 (R) |
| 3 | NEt3 | 95 | 99 (R) |
| 4d) | NEt3 | <5 | - |
| 5e) | NEt3 | 92 | 00 (R) |
| 6f) | NEt3 | 58 | 99 (R) |
General procedure: 0.20 mmol cyclohexenone, 0.24 mmol PhB(OH)2, 0.006 mmol PQS-BINAP-Rh, 0.60 mmol base, and 0.4 mL H2O (0.5 M).
Yield of isolated product.
Determined by HPLC on chiral stationary phase.
0.5 equiv base.
Reaction run in 0.8 mL H2O (0.25 M).
1 mol % of PQS-BINAP-Rh.
