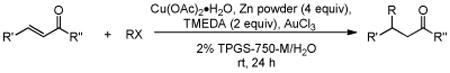
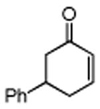
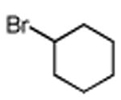
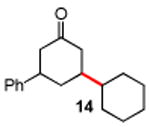
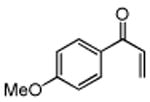
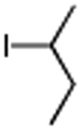
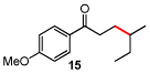
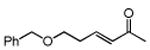
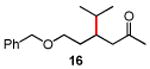
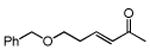
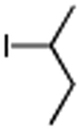
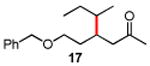
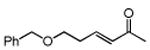
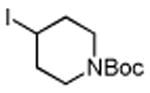
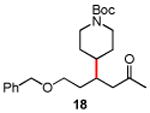
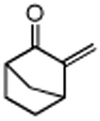
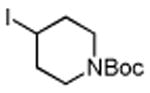
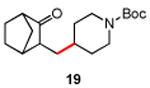
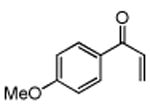
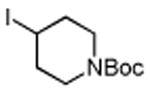
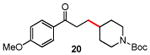
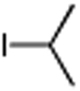Table 3. Conjugate Addition Reactions of Secondary Alkyl Halides to Enonesa.
Conditions: alkyl-X (3 equiv), Zn powder (X = I) or Zn dust (X = Br) (4 equiv), TMEDA (2 equiv), 3 mol % [Cu], 5 mol % AuCl3, 0.5 mL 2 wt %-TPGS-750-M/H2O, rt, 24h.
Isolated, chromatographically purified.
Tetraethyl derivative of TMEDA used.