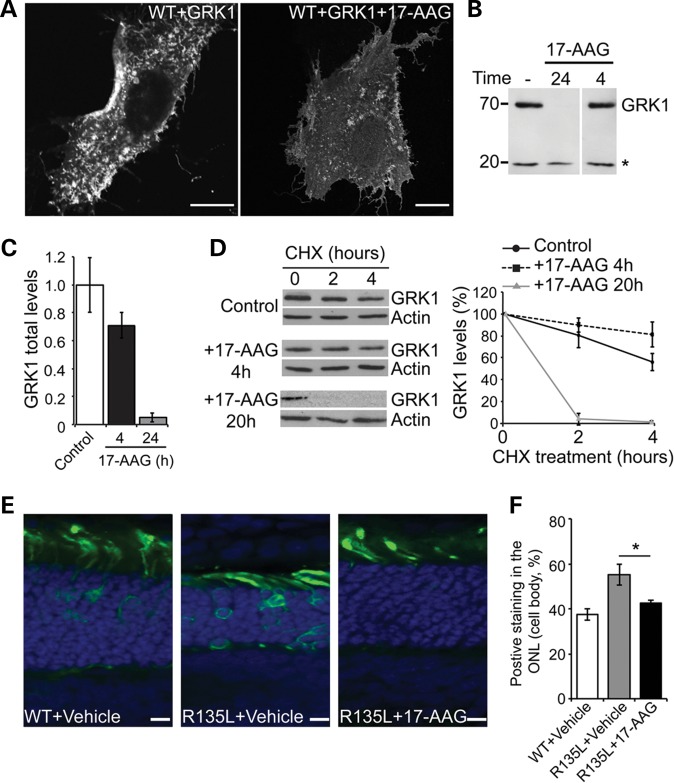
Figure 5.
Hsp90 inhibition blocks R135L:arrestin recruitment through GRK1 (A) SK-N-SH cells co-transfected with WT-GFP rod opsin and FLAG-GRK1 and treated with 17-AAG (1 μm) or vehicle for 24 h. Scale bars: 10 μm. (B) Western blot of FLAG-GRK1 expression following 17-AAG treatment. SK-N-SH cells were transfected with FLAG-GRK1 and treated with 17-AAG (1 μm) for 24 or 4 h as indicated. Ten micrograms of soluble protein were resolved and detected using anti-FLAG mAb. Asterisk highlights a non-specific band used as a loading control. (C) Quantification of total FLAG-GRK1 levels, normalized relative to loading control, after incubation with 17-AAG for the indicated times. Values are mean ± SEM (n > 6). (D) Degradation of GRK1 in the presence of 17-AAG. Left panel, western blot for FLAG-GRK1 and actin of SK-N-SH cell lysates treated with 17-AAG for the indicated times prior to addition of CHX (50 μg/ml) for the indicated times. Exposures have been adjusted so that time 0 is approximately equivalent. Right panel shows quantification of GRK1 levels normalized to time 0. Values are mean ± SEM (n ≥ 3). (E) In vivo electroporation of WT and R135L rod opsin. WT-GFP or R135-GFP (green), as indicated, was injected subretinally with vehicle or 17-AAG (20 mg/ml) and electroporated in neonatal SD rats. Eyes were analyzed 16 days postelectroporation. Nuclei were stained with DAPI. Scale bars: 10 μm. (F) Quantitation of over 100 transfected cells (n = 4) showed WT-GFP was mainly present in the ROS, whereas R135L-GFP in the presence of vehicle was observed throughout the photoreceptor cell layer. Treatment with 17-AAG partially shifted the mutant protein to the ROS and reduced cell body staining. * P < 0.05, Student's t-test.