Table 2. Relative energies of the eight diastereomeric transition states for the benzoin condensation with catalyst 2.
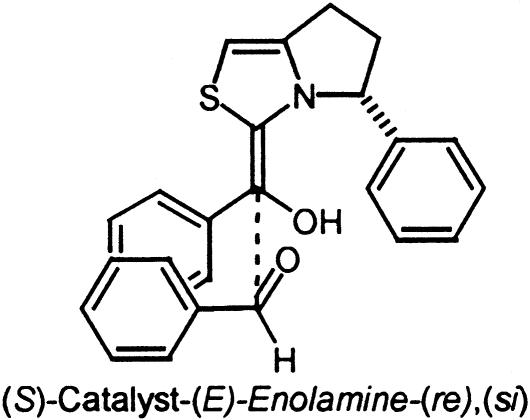 Transition-state geometry* |
|||||
|---|---|---|---|---|---|
| Catalyst | Enolamine | Enolamine face | Aldehyde face | Product configuration | ΔGrel, kcal/mol† |
| S | E | re | si [TS3]‡ | R | 0 |
| S | E | si | re [TS4]‡ | S | 0.7 |
| S | Z | si | re | S | 0.8 |
| S | Z | re | re | S | 0.8 |
| S | E | si | si | R | 1.6 |
| S | E | re | re | S | 1.7 |
| S | Z | si | si | R | 5.9 |
| S | Z | re | si | R | 9.8 |
See Data Set 1 for figures of structures and Cartesian coordinates.
Relative energies obtained by single-point B3LYP/6-31G(d) calculations.
Depicted in Fig. 6.
