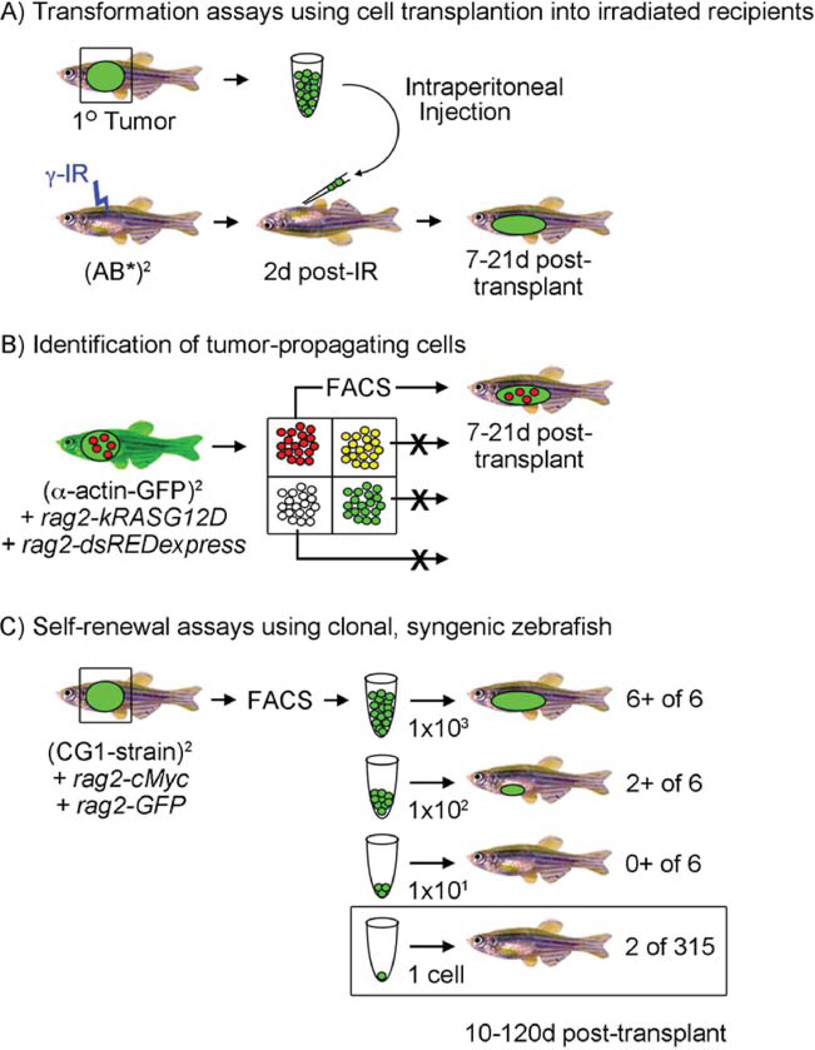
Fig. 3.
Cell transplantation assays in zebrafish. (A) Establishing malignancy by cell transplantation into irradiated recipients. A GFP+ primary tumor can be excised and individual cells isolated by disaggregation and filtering. Cells are induced into irradiated recipient animals (23 Gy, 2 days post-IR) and recipients assessed for engraftment from 7 to 21 days post-transplantation. (B) Identification of tumor-propagating cells. In this published example (Langenau et al., 2007), rag2-kRASG12D was co-injected with rag2-dsREDexpress into one-cell stage alpha-actin-GFP+ animals. Because the rag2 promoter expresses in satellite cells and early myoblasts while alpha-actin is expressed in late myoblasts and fused fibers, four cell populations could be identified following tumor resection, disaggregation, and fluorescent activated cell sorting (FACS). Each cell population is introduced into irradiated recipient animals and assessed for the ability to remake tumor. In this case, only the rag2-dsREDexpress+/alpha-actin-GFP-negative cells were capable of engraftment. (C) Self-renewal and single cell transplant assays using clonal, syngeneic zebrafish. In this example based onSmith et al. (2010), fluorescently labeled T-ALLs were created by microinjecting rag2-mouse cMyc (rag2-cMyc) and rag2-GFP into one-cell stage CG1-strain, syngeneic zebrafish. A subset of animals develop fluorescent-labeled T-ALLs that can be FACS sorted and transplanted at limiting dilution into CG1 recipient animals. Six animals per group were monitored for T-ALL onset up to 120 days post-transplantation. Limiting dilution cell transplantation assays accurately determine the number of leukemia-initiating cells contained within the bulk of the tumor mass and have been reviewed previously (Ignatius and Langenau, 2009). A single cell can also be transplanted into recipient fish, but fewer animals develop disease. (See color plate.)