Abstract
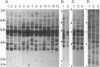
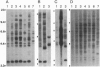
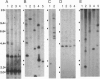
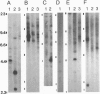
The jaagsiekte sheep retrovirus (JSRV), which appears to be a type B/D retrovirus chimera, has been incriminated as the cause of ovine pulmonary carcinoma. Recent studies suggest that the sequences related to this virus are found in the genomes of normal sheep and goats. To learn whether there are breeds of sheep that lack the endogenous viral sequences and to study their distribution among other groups of mammals, we surveyed several domestic sheep and goat breeds, other ungulates, and various mammal groups for sequences related to JSRV. Probes prepared from the envelope (SU) region of JSRV and the capsid (CA) region of a Peruvian type D virus related to JSRV were used in Southern blot hybridization with genomic DNA followed by low- and high-stringency washes. Fifteen to 20 CA and SU bands were found in all members of the 13 breeds of domestic sheep and 6 breeds of goats tested. There were similar findings in 6 wild Ovis and Capra genera. Within 22 other genera of Bovidae including domestic cattle, and 7 other families of Artiodactyla including Cervidae, there were usually a few CA or SU bands at low stringency and rare bands at high stringency. Among 16 phylogenetically distant genera, there were generally fewer bands hybridizing with either probe. These results reveal wide-spread phylogenetic distribution of endogenous type B and type D retroviral sequences related to JSRV among mammals and argue for further investigation of their potential role in disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Wilson G. L., Todaro G. J. Skunks have gene sequences in their cellular DNA related to squirrel monkey retrovirus: transmission between species of a new world primate endogenous type D retrovirus. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1363–1369. doi: 10.1016/0006-291x(78)91286-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Tronick S. R., Aaronson S. A. Immunological relationships of an endogenous guinea pig retrovirus with prototype mammalian type B and type D retroviruses. J Virol. 1980 Jan;33(1):522–530. doi: 10.1128/jvi.33.1.522-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De las Heras M., Sharp J. M., Garcia de Jalon J. A., Dewar P. Enzootic nasal tumour of goats: demonstration of a type D-related retrovirus in nasal fluids and tumours. J Gen Virol. 1991 Oct;72(Pt 10):2533–2535. doi: 10.1099/0022-1317-72-10-2533. [DOI] [PubMed] [Google Scholar]
- Demartini J. C., Rosadio R. H., Lairmore M. D. The etiology and pathogenesis of ovine pulmonary carcinoma (sheep pulmonary adenomatosis). Vet Microbiol. 1988 Jul;17(3):219–236. doi: 10.1016/0378-1135(88)90067-3. [DOI] [PubMed] [Google Scholar]
- Hecht S. J., Carlson J. O., DeMartin J. C. Analysis of a type D retroviral capsid gene expressed in ovine pulmonary carcinoma and present in both affected and unaffected sheep genomes. Virology. 1994 Jul;202(1):480–484. doi: 10.1006/viro.1994.1366. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Irwin D. M., Kocher T. D., Wilson A. C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991 Feb;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Perl A. Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J. 1992 May;6(8):2537–2544. doi: 10.1096/fasebj.6.8.1592206. [DOI] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Günzburg W. H. Current perspectives in the biology of mouse mammary tumour virus. Virus Res. 1987 Aug;8(2):81–102. doi: 10.1016/0168-1702(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Golub M. M., Stephenson J. R., Aaronson S. A. Distribution and expression in mammals of genes ralated to an endogenous type C RNA virus of Odocoileus hemionus. J Virol. 1977 Jul;23(1):1–9. doi: 10.1128/jvi.23.1.1-9.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Berger S. L., Kimmel A. R. Molecular hybridization of immobilized nucleic acids: theoretical concepts and practical considerations. Methods Enzymol. 1987;152:399–407. doi: 10.1016/0076-6879(87)52046-8. [DOI] [PubMed] [Google Scholar]
- York D. F., Vigne R., Verwoerd D. W., Querat G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J Virol. 1992 Aug;66(8):4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York D. F., Williamson A., Barnard B. J., Verwoerd D. W. Some characteristics of a retrovirus isolated from transformed bovine cells. Virology. 1989 Aug;171(2):394–400. doi: 10.1016/0042-6822(89)90607-7. [DOI] [PubMed] [Google Scholar]