Abstract
Two phosphorylases have been found in the endosperm of Zea mays. Phosphorylase I is found through all stages of endosperm development and seed germination investigated. The other enzyme, phosphorylase II appears only at the stage of rapid starch biosynthesis and is not found during germination. At 22 days after pollination, the activity of phosphorylase II is 10 times that of phosphorylase I. These 2 phosphorylases are separable by column chromatography and behave differently in several respects.
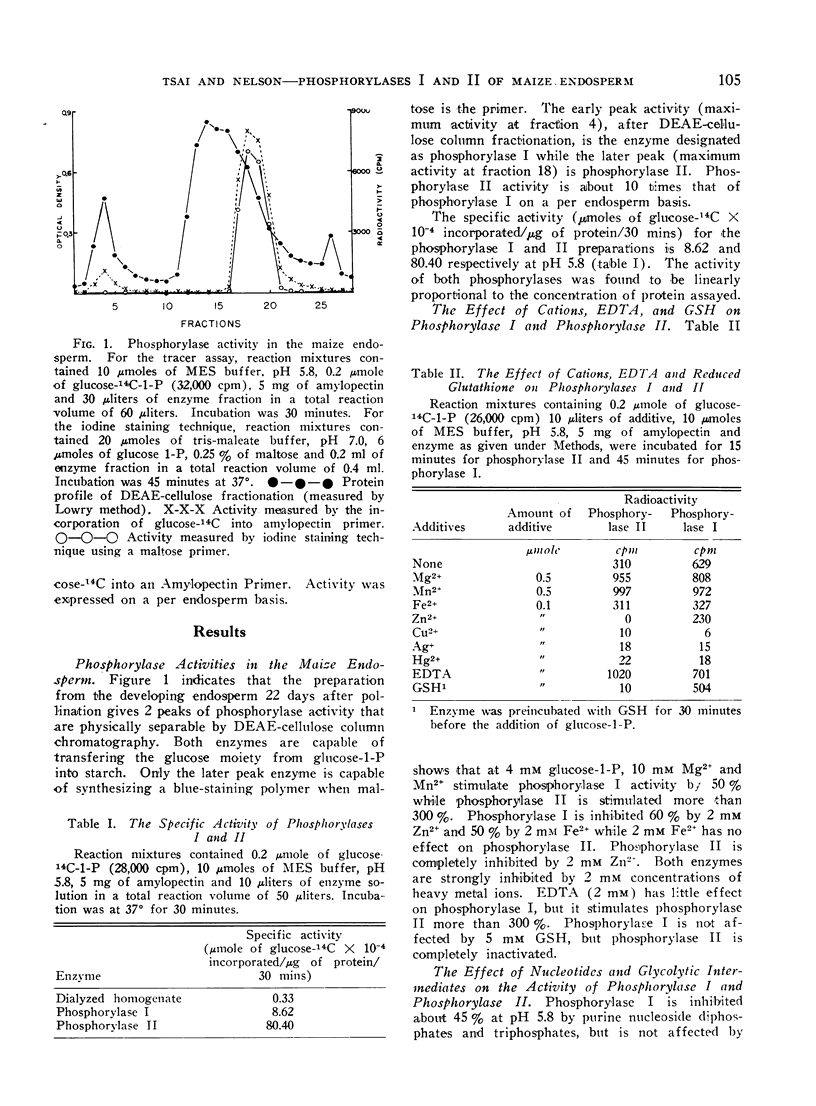
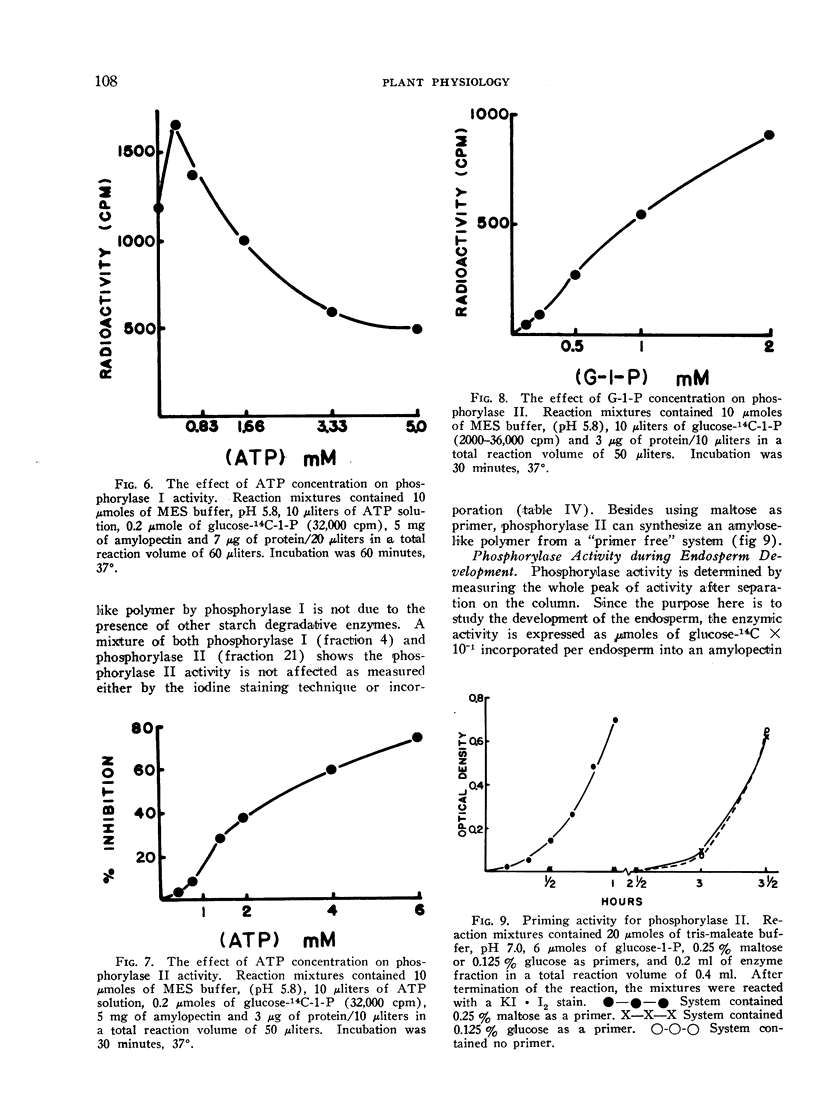
Phosphorylase I cannot utilize maltose as a primer while phosphorylase II does so readily. Furthermore, phosphorylase II can synthesize an amylose-like polymer from a “primer free” system after a lag phase.
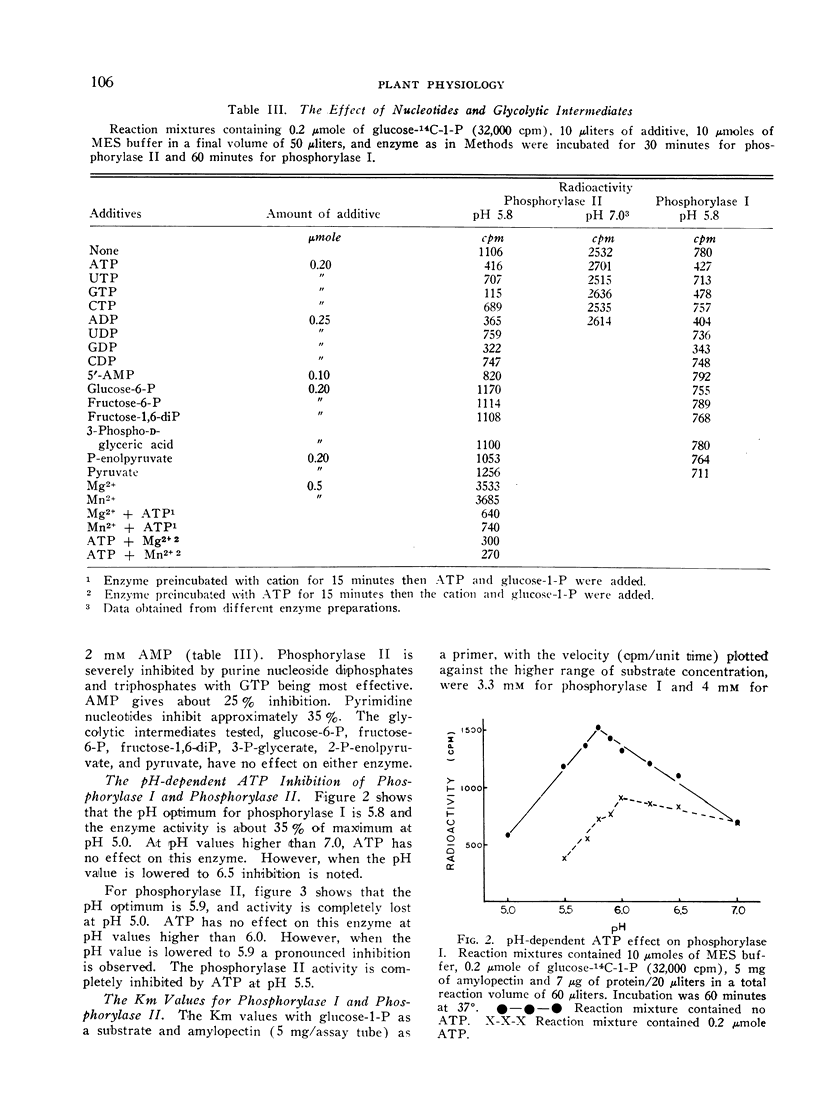
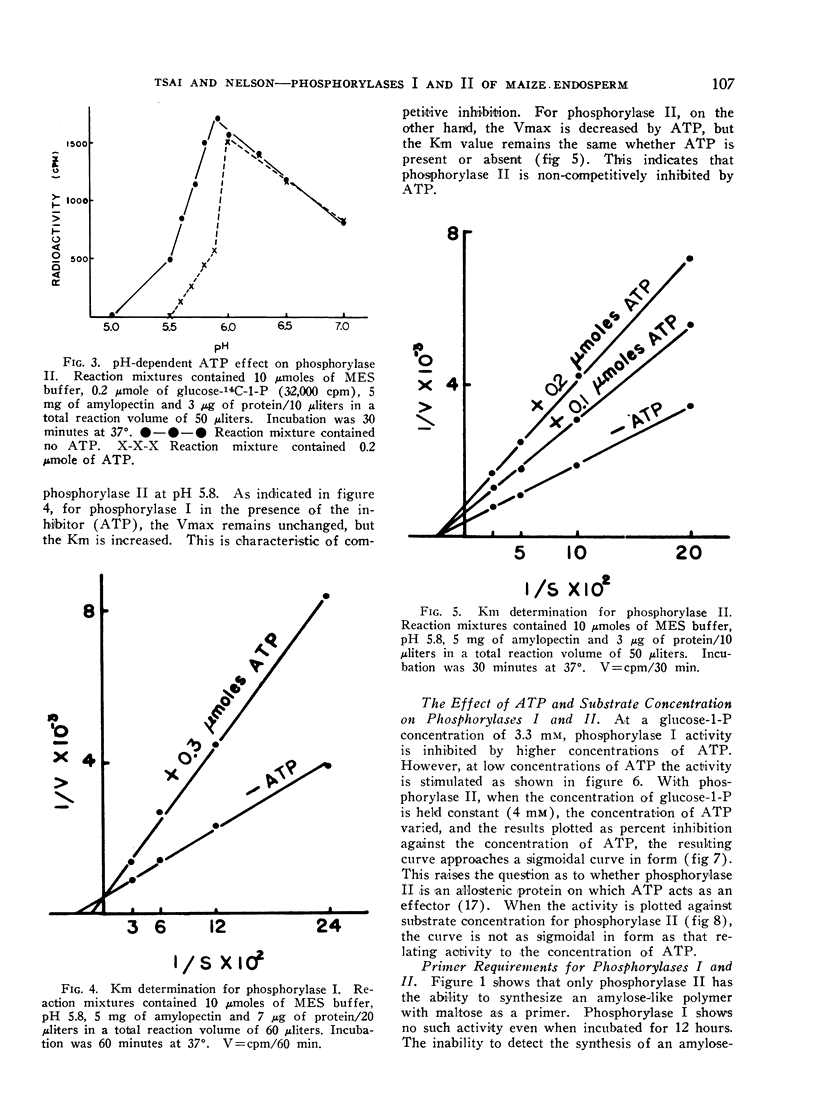
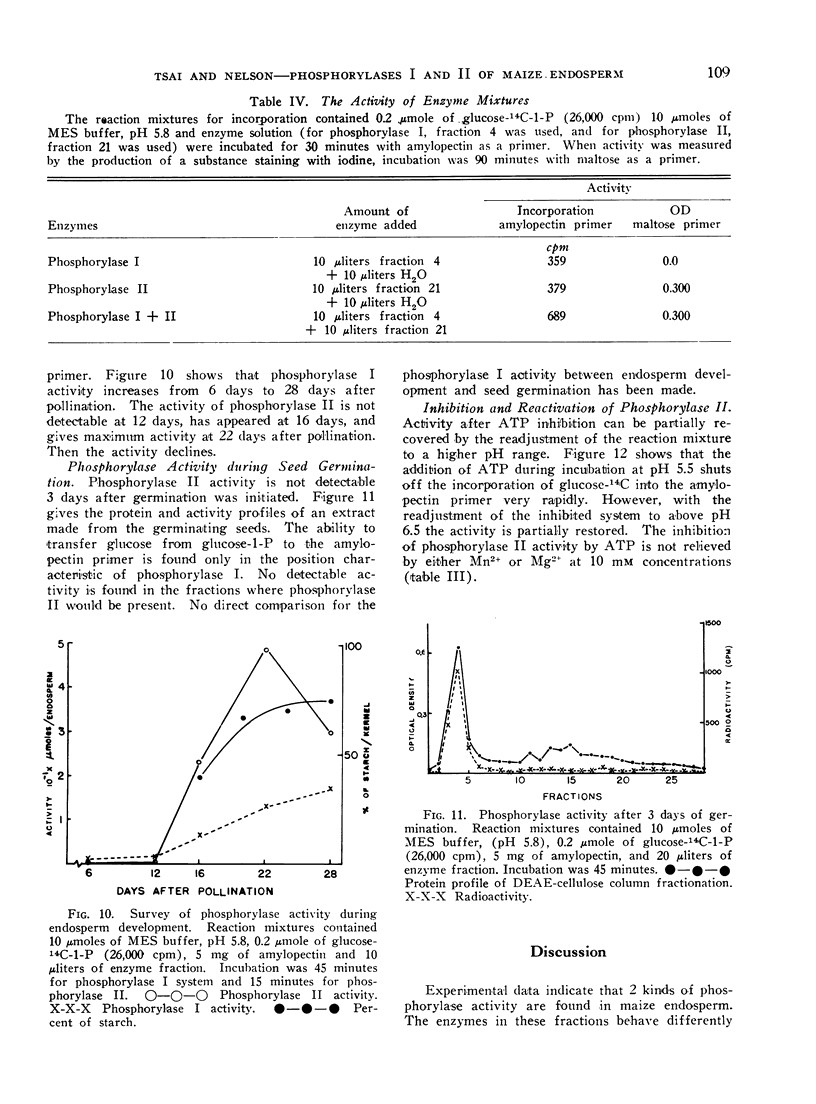
Phosphorylase II is inhibited severely at pH 5.8 by ATP, GTP, ADP, and GDP, and less drastically by UTP, CTP, UDP and CDP. Phosphorylase I is somewhat inhibited by purine nucleotides but not by pyrimidine nucleotides. In all cases, the inhibition is pH-dependent. Phosphorylase I is inhibited competitively by ATP while phosphorylase II is inhibited non-competitively.
Phosphorylase II is markedly stimulated by 10 mm Mg2+ and by 2 mm ethylenediamine tetraacetic acid while phosphorylase I is relatively little affected.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
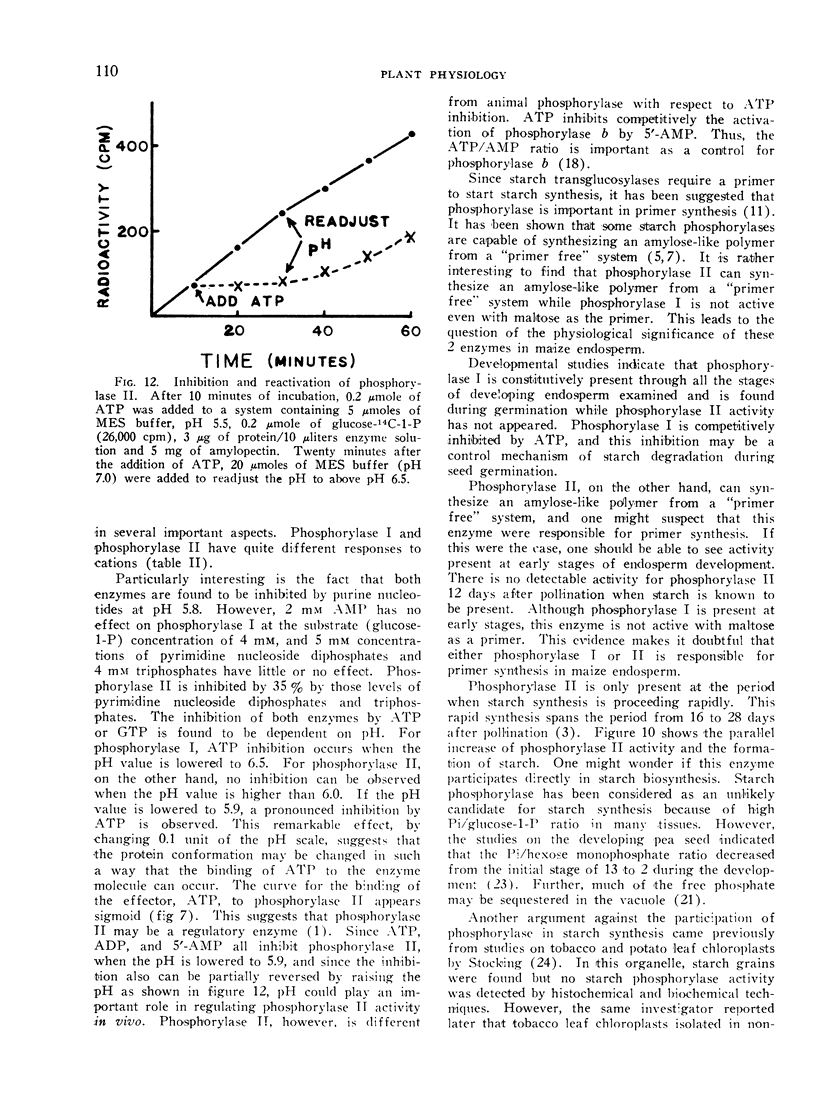
- Creech R G. Genetic Control of Carbohydrate Synthesis in Maize Endosperm. Genetics. 1965 Dec;52(6):1175–1186. doi: 10.1093/genetics/52.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart M. H., Siminovitch D., Briggs D. R. Studies on the Chemistry of the Living Bark of the Black Locust in Relation to its Frost Hardiness. VIII. Possible Enzymatic Processes Involved in Starch-Sucrose Interconversions. Plant Physiol. 1954 Sep;29(5):407–413. doi: 10.1104/pp.29.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILLINGWORTH B., BROWN D. H., CORI C. F. The de novo synthesis of polysaccharide by phosphorylase. Proc Natl Acad Sci U S A. 1961 Apr 15;47:469–478. doi: 10.1073/pnas.47.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., PARMEGGIANI A. REGULATION OF GLYCOGENOLYSIS IN MUSCLE. 3. CONTROL OF MUSCLE GLYCOGEN PHOSPHORYLASE ACTIVITY. J Biol Chem. 1964 Aug;239:2440–2445. [PubMed] [Google Scholar]
- NELSON O. E., RINES H. W. The enzymatic deficiency in the waxy mutant of maize. Biochem Biophys Res Commun. 1962 Oct 31;9:297–300. doi: 10.1016/0006-291x(62)90043-8. [DOI] [PubMed] [Google Scholar]
- NELSON O. E., TSAI C. Y. GLUCOSE TRANSFER FROM ADENOSINE DIPHOSPHATE-GLUCOSE TO STARCH IN PREPARATIONS OF WAXY SEEDS. Science. 1964 Sep 11;145(3637):1194–1195. doi: 10.1126/science.145.3637.1194. [DOI] [PubMed] [Google Scholar]
- RECONDO E., LELOIR L. F. Adenosine diphosphate glucose and starch synthesis. Biochem Biophys Res Commun. 1961 Nov 1;6:85–88. doi: 10.1016/0006-291x(61)90389-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan P. N., Srivastava L. M. Search for uridine diphosphate glucose-starch synthetase and phosphorylase activity in polyfructosan bearing tissues. Indian J Biochem. 1964 Sep;1(3):133–136. [PubMed] [Google Scholar]
- WHELAN W. J., BAILEY J. M. The action pattern of potato phosphorylase. Biochem J. 1954 Dec;58(4):560–569. doi: 10.1042/bj0580560. [DOI] [PMC free article] [PubMed] [Google Scholar]


