Abstract
Background:
Aquaporins (AQPs), members of a superfamily of transmembrane channel proteins, are ubiquitous in all domains of life. They fall into a number of branches that can be functionally categorized into two major sub-groups: i) orthodox aquaporins, which are water-specific channels, and ii) aquaglyceroporins, which allow the transport of water, non-polar solutes, such as urea or glycerol, the reactive oxygen species hydrogen peroxide, and gases such as ammonia, carbon dioxide and nitric oxide and, as described in this review, metalloids.
Scope of Review:
This review summarizes the key findings that AQP channels conduct bidirectional movement of metalloids into and out of cells.
Major Conclusions:
As(OH)3 and Sb(OH)3 behave as inorganic molecular mimics of glycerol, a property that allows their passage through AQP channels. Plant AQPs also allow the passage of boron and silicon as their hydroxyacids, boric acid (B(OH)3) and orthosilicic acid (Si(OH)4), respectively. Genetic analysis suggests that germanic acid (GeO2) is also a substrate. While As(III), Sb(III) and Ge(IV) are toxic metalloids, borate (B(III)) and silicate (Si(IV)) are essential elements in higher plants.
General Significance:
The uptake of environmental metalloids by aquaporins provides an understanding of (i) how toxic elements such as arsenic enter the food chain; (ii) the delivery of arsenic and antimony containing drugs in the treatment of certain forms of leukemia and chemotherapy of diseases caused by pathogenic protozoa; and (iii) the possibility that food plants such as rice could be made safer by genetically modifying them to exclude arsenic while still accumulating boron and silicon.
Keywords: Metalloid, antimonite, arsenite, arsenate, boron, silicon, germanium, aquaglyceroporins, channel, Fps1, AQP7, AQP9, GlpF, LmAQP1, MAP kinase
Introduction
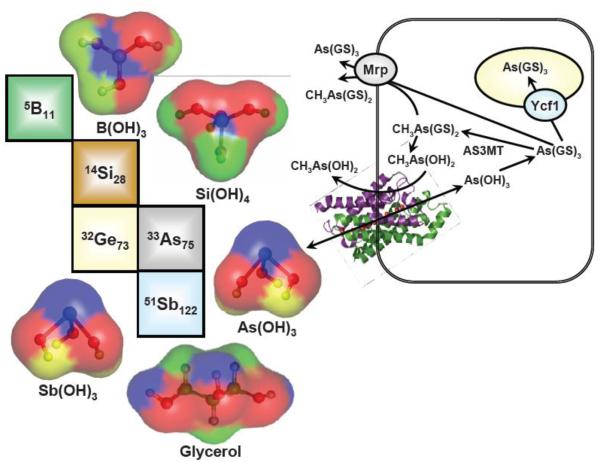
The term ‘metalloid’ is frequently used to describe a chemical element that exhibits physical and chemical properties that are intermediate between metals and nonmetals. General characteristics of metalloids include lustrous, brittle solids with intermediate to good electrical conductivity. They generally form amphoteric to weakly acidic oxides; have electronegativity values close to 2.0, and ionization energies averaging around 200 kcal/mol. The six elements that are generally recognized as metalloids are arsenic (As), antimony (Sb), boron (B), germanium (Ge), silicon (Si) and tellurium (Te). The natural abundance of these metalloids varies considerably. Silicon is the second most abundant element in the Earth’s crust after oxygen and constitutes approximately 28% of the crust by weight; in contrast, tellurium is present in trace levels bordering on 1 × 10−7 %. The role of the metalloids in biological processes ranges from essential to toxic. For metalloids to affect biological systems, they must move across the cellular membrane and accumulate in cells. This review discusses the role of aquaporins in conducting the bidirectional movement of the metalloids B, Ge, Si, As and Sb across cellular membranes (Fig. 1).
Fig. 1. Periodic table of metalloids.
The hydroxyacids of lower oxidation state metalloid species (B(III), Si(IV), Ge(IV), As(III) and Sb(III)) are substrates of aquaglyceroporin (AQP) channels in bacteria, protozoans, fungi, plants and animals. Like glycerol, they are uncharged polar molecules with volumes small enough to fit through the approximately 5 Å diameter opening of the channel. Shown is a composite bacterial-yeast-mammalian cell with As(OH)3 entering via the E. coli GlpF AQP (the structure is PDB 1LDA). Inside the cell, the metalloid reacts with three GSH molecules to form the major cytosolic species, As(GS)3. This molecule is detoxified by removal from the cytosol. In fungi and plants, As(GS)3 (or other thiol derivatives such as phytochelatin conjugates) are pumped into the vacuole by ABC transporters such as the yeast Ycf1, or, in animals, into bile or blood by related MRP pumps. As(GS)3 can also be detoxified by methylation catalyzed by As(III) S-adenosylmethionine methyltransferases (AS3MT in animals, ArsM in microbes). The product of the first round of methylation, CH3As(GS)2 is a substrate of MRPs, and, after hydrolysis to CH3As(OH)2, is a substrate of AQPs.
2. Biological associations of arsenic and antimony
Arsenic is ubiquitous in the Earth's crust and enters the food chain from drinking water that has flown through arsenic-containing soil. Arsenic, a Group 1 human carcinogen, is the most ubiquitous environmental toxin and carcinogen according to the U.S. Environmental Protection Agency (EPA) and Agency for Toxic Substances and Disease Registry (ATSDR), which ranks arsenic at the top of the U.S. Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/SPL/index.html). The EPA asserts that it pervades our drinking water [1] and imperils the safety of our food supply [2]. It enters our water and food supply in many ways, including natural geochemical sources, herbicides, antimicrobial growth promoters, wood preservatives, burning of coal and other sources. Long-term exposure to arsenic in drinking water and food supply, as well as occupational exposure, causes cancer [3-7]. Arsenic contamination of groundwater in Bangladesh has been called the largest mass poisoning of a population in history [8]. In humans, exposure is a major contributor to arsenic-related diseases [4, 9], including bladder [10], lung [11] and skin cancers [12]. Bladder cancer, a major form associated with exposure, is the 10th most common cancer worldwide, accounting for an estimated 261,000 new cases diagnosed and 115,000 deaths each year. In 2002, the EPA’s Maximum Containment Level (MCL) was lowered from 50 to 10 parts per billion (ppb), but in the U.S. more than 6 million people have drinking water above 10 ppb, and 2.5 million people have levels above 25 ppb, which increases the lifetime risk of dying from bladder cancer from drinking 1 liter per day of water to approximately 1 per 1000 persons [13]. More than 350,000 people in the United States drink water containing more than 50 ppb arsenic, which increases the lifetime risk to as much as 13 per 1000 persons. In addition, arsenic exposure during pregnancy contributes to low birth weight and fetal loss, as well as delayed infant development [14].
The two biologically relevant oxidation states of arsenic are the pentavalent arsenate (As(V)) and trivalent arsenite (As(III)) forms. In solution at physiological pH, the pentavalent form exists as the oxyanion As(V), which is a substrate analogue of phosphate, and hence a competitive inhibitor for enzymes that either use phosphate or have phosphorylated intermediates. Trivalent As(III) and its methylated forms are much more toxic than As(V) and are primarily responsible for the biological effects of this metalloid. Trivalent inorganic and organic arsenicals have the propensity to form strong, nearly covalent bonds with the thiolates of closely spaced cysteine residues, thereby inhibiting the function of many proteins. They also react with other cellular thiols such as reduced glutathione (GSH), raising the intracellular redox potential, and leading to oxidative damage.
Although antimonials are less abundant in the environment than arsenicals, their chemical properties are very similar. Exposure to antimony can occur from both natural sources and industrial activities. Antimony compounds are widely used in flame-retardant applications, as alloying metal, ceramics and plastics, and in the microelectronics industry. The primary effects from chronic exposure to antimony in humans are respiratory problems, lung damage, cardiovascular effects, gastrointestinal disorders, and adverse reproductive outcome. Antimony has not as yet been classified as a human carcinogen by either the U.S. Department of Health and Human Services or the EPA (http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=331&tid=58).
In spite of their toxicity, arsenic and antimony compounds are also used as chemotherapeutic agents. Arsenic containing drugs are currently used for the treatment of acute promyelocytic leukemia (APL) [15]. Melarsoprol, an organoarsenical, is the first line of treatment against late stage sleeping sickness [16]. Pentavalent antimony-containing drugs such as Pentostam and Glucantime are the treatment of choice for Leishmania infections (leishmaniasis) [17].
3. Aquaglyceroporins conduct As(III) and Sb(III)
To act as a drug or poison, arsenic and antimony compounds must accumulate in cells. In this review, we will summarize the role of AQPs in transport of these metalloids and the functional consequences in human disease. Additional information about metalloid transport pathways can be found in several recent reviews [18, 19]
3.1. Metalloid channels in bacteria
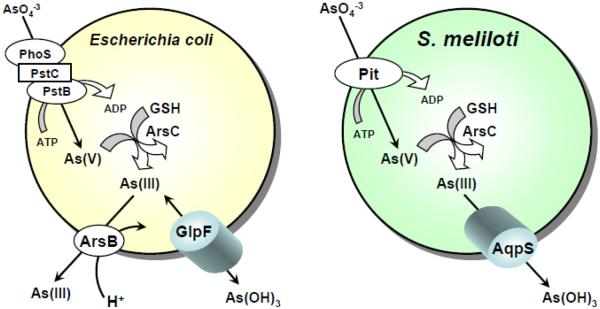
As a substrate analogue of phosphate, arsenate is accumulated in most cells by phosphate transporters. In Escherichia coli there are two phosphate transporters, Pit and Pst [20], both of which catalyze arsenate uptake [21, 22] (Fig. 2). Given the structural similarities between arsenate and phosphate, As(V) is adventitiously taken up by phosphate transporters in most organisms, including humans.
Fig. 2. E. coli GlpF and S. meliloti AqpS are bidirectional As(III) channels with different physiological roles.
Arsenate, which is chemically similar to phosphate, is taken up by phosphate transporters in both E. coli and S. meliloti. In both organisms, As(V) is reduced by ArsC to As(III). In both, internally-generated As(III) is removed from the cytosol. In E. coli efflux is an active, energy-dependent reaction catalyzed by the As(OH)3/H+ antiporter ArsB. In S. meliloti, the AqpS channel facilitates downhill efflux of internally-generated As(III). In both cases this results in resistance to arsenate. E. coli takes up As(III) by GlpF, which is immediately extruded by ArsB, conferring resistance to As(III). In contrast, As(III) uptake by AqpS in S. meliloti results in lethality. Thus AqpS produces sensitivity to extracellular arsenite but resistance to intracellularly-generated arsenite. This may be an adaptation of the microaerophilic plant symbiont for arsenic tolerance under conditions of nitrogen fixation.
The pathways for As(III) uptake were identified later (Fig. 2). The first identification of a cellular transporter for As(III) and Sb(III) accumulation was in E. coli [23]. Cells were subjected to random TnphoA mutagenesis, which inserts into the genes for membrane proteins to generate blue colonies because the gene for alkaline phosphatase (phoA) is exposed to the periplasmic space, enriching for insertions in the genes for transporters. An Sb(III)-resistant strain was isolated [23], which exhibited a 90% reduction in the rate of uptake of both As(III) and glycerol uptake [24]. Sequence analysis showed that the TnphoA insertion was within the glpF gene, which encodes the aquaglyceroporin, GlpF. These results suggested that in solution GlpF recognizes either As(III) or Sb(III) as an inorganic molecular mimic of glycerol . Although the mutant was resistant to Sb(III), it retained some sensitivity to As(III), indicating that there may be one or more additional uptake systems that account for the residual 10% of As(III) uptake.
GlpF is a member of the major intrinsic protein (MIP) superfamily that allow the transport of water and small solutes such as glycerol and urea by an energy independent mechanism. Members of the MIP superfamily fall into a number of branches, but the two main evolutionary groups are the aquaporins or water specific channels, and the aquaglyceroporins, which allow the transport of water, glycerol, and other small, uncharged solutes [25, 26]. Both groups are found in all living organisms. These two groups represent the majority of MIPs in mammals, including humans [27]. Plants appear to have more MIPs than other organisms, with additional major subfamilies identified by phylogeny [28, 29]. In plants, metalloids are conducted by the Nodulin26-like MIPS (or NIPs) channels, which are a separate phylogenetic branch from GlpF. The other groups differ in substrate specificity, physiological function and tissue distribution. These include the tonoplast MIPs or TIPS, PIPs or plasma membrane MIPs, GlpF-like MIPs or GIPs, Small basic MIPS or SIPS and X MIPS or XIPs.
How can As(III), which was often considered to be the anion arsenite in solution, be taken up by GlpF, a channel for neutral species? Arsenite in water has a pKa of 9.2, and it would be expected to exist as a hydroxyacid in solution. To examine this question, X-ray absorption spectroscopy (XAS) was used to determine the nearest neighbor coordination environment of As(III) under a variety of solution conditions [30]. Extended X-ray Absorption Fine Structure (EXAFS) analysis demonstrated three 3 As-O bonds at an average bond length of 1.77 Å, establishing that the undissociated oxyacid, As(OH)3, is the major arsenite species in solution at neutral pH [30]. This is the most likely the solution form of the chemotherapeutic drug arsenic trioxide as well. Additionally, structural, thermodynamic, and electrostatic comparison of As(OH)3 and Sb(OH)3 showed very strong similarities to each other and the conformation of the glycerol molecule. Both As(OH)3 and Sb(OH)3 showed a similar charge distribution and a slightly smaller volume than glycerol (Fig. 1). The smaller size of As(III) and Sb(III) may be an advantage for the metalloids to navigate through the narrowest region of the GlpF channel [31].
While the E. coli GlpF channel facilitates adventitious uptake of As(III) and Sb(III) and causes the cells to become sensitive to the metalloids, the legume symbiont Sinorhizobium meliloti, a Gram-negative nitrogen-fixing bacterium, employs an aquaglyceroporin as a novel route for arsenic efflux and resistance (Fig. 2). When S. meliloti is exposed to environmental As(V), As(V) enters the cell through the phosphate transport system and is reduced to As(III) by the cytosolic arsenate reductase, ArsC. Internally-generated As(III) flows out of the cell by downhill movement through AqpS, an aquaglyceroporin orthologue [32]. Together AqpS and ArsC form an unusual pathway of As(V) detoxification in S. meliloti. Thus, depending upon the concentration gradient, aquaglyceroporins can facilitate movement of arsenite either into or out of cells. This was the first report of an aquaglyceroporin with a physiological role in arsenic detoxification.
A variant of the above scheme is observed in Salinispora tropica, a marine actinomycete. The S. tropica genome encodes for a novel fusion protein (Strop634), which consists of two domains - an N-terminal aquaglyceroporin derived channel domain attached by a linker region to a C-terminal arsenate reductase (ArsC) domain [33]. Thirty nine percent of the residues lining the interior of Strop634 channel are polar amino acids (by comparison, other AQPs such as GlpF have only about 18% polar residues), which probably accounts for its enhanced selectivity for arsenite and low permeability for either water or glycerol. The Strop634 fusion assembly is a unique feat of evolution in assembling an As(III)-generating enzyme and an As(III) selective channel in one structure. The advantage of such a system over S. meliloti is that the As(III) generated by the ArsC reductase activity is compartmentalized and channeled out of the cell by the same protein, thereby facilitating removal of the toxic metalloid in an energy-efficient manner.
3.2. Metalloid channels in yeast
The GlpF homologue, Fps1, mediates the influx of As(III), Sb(III), and B(III) in Saccharomyces cerevisiae [34-36]. Fps1 is a plasma membrane glycerol channel with a critical role in osmoregulation. It is closed under hyperosmotic conditions, permitting glycerol accumulation and turgor recovery, whereas, a hypo-osmotic shock triggers channel opening, and a rapid release of glycerol to prevent cell bursting [37].
S. cerevisiae with an FPS1 deletion show improved tolerance to both As(III) and Sb(III) [34]. Conversely, cells expressing a constitutively open form of Fps1 channel are highly sensitive to both As(III) and Sb(III). Under high osmolarity conditions, when the Fps1 channel is closed, wild-type cells show the same degree of As(III) and Sb(III) tolerance as the fps1Δ mutant. Direct transport assays also demonstrate that As(III) uptake is mediated by Fps1. Fps1-mediated arsenic uptake is down-regulated by the mitogen-activated protein kinase (MAPK) homologue Hog1 [38]. Cells with elevated Hog1 activity display improved metalloid tolerance, whereas cells impaired in Hog1 function are hypersensitive to As(III) and Sb(III). Direct transport assays show that As(III) uptake is higher in hog1Δ mutant than in the wild type yeast cells. Hog1 phosphorylates a threonine residue (Thr231) at the N-terminal extension of Fps1, resulting in reduced metalloid influx. Therefore, inactivation of Hog1 reduces Fps1 phosphorylation, ensuing an increased As(III) influx through Fps1 and hypersensitivity to metalloid.
The paralogous S. cerevisiae proteins, Rgc1 (Ypr115w) and Rgc2 (Ask10), are positive regulators of glycerol efflux through Fps1 [39]. Rgc1 and Rgc2 are members of the family of pleckstrin homology domain proteins. In comparison to wild type yeast cells that are sensitive to As(III), either the rgc1Δ or rgc2Δ mutants show resistance to the metalloid, suggesting that Rgc1 and Rgc2 are positive regulators of Fps1 channel activity. While a hog1Δ mutant is hypersensitive to As(III) toxicity, the rgc1Δrgc2Δhog1Δ triple mutant is resistant to As(III), suggesting that Hog1 exerts its negative effect on Fps1 by inhibiting Rgc1 and Rgc2. Rgc2 is phosphorylated in response to various Fps1-regulatory stress conditions, such as arsenic stress, hypoosmotic and hyperosmotic shock. Rgc2 also undergoes Hog1-dependent basal phosphorylation. It is likely that Fps1 channel activity is down-regulated directly through phosphorylation of Thr231 by Hog1, and indirectly through phosphorylation of Rgc1 and Rgc2.
Expression of FPS1 is rapidly repressed upon As(III) or Sb(III) addition to the medium, most likely to prevent metalloid uptake [34]. However, upon prolonged incubation with metalloids, expression of FPS1 is up-regulated, presumably to export arsenic out of the cell [40]. Like S. meliloti AqpS [32], Fps1 also has a physiological role in mediating As(V) tolerance and facilitates extrusion of As(III) out of the cells [40]. When yeast cells are exposed to arsenate, pentavalent arsenic entering the cells via the phosphate transporters is reduced to As(III) by the arsenate reductase Acr2. The internally generated As(III) is either actively extruded by the arsenite permease Acr3 [40-42] or diffuses out of the cells through the Fps1 channel. Therefore yeast Fps1 is also a bidirectional arsenite channel [40]
3.3 As(III) transport by aquaglyceroporins and hexose permeases in humans and other vertebrates
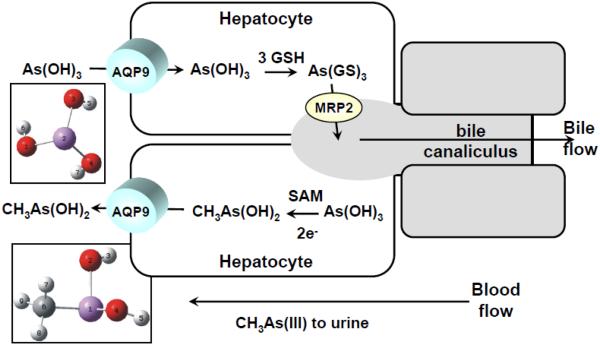
Thirteen AQPs have been identified in mammals, of which four are classical aquaglyceroporins: AQP3, 7, 9, and 10 [43]. A yeast strain lacking Fps1 was used to functionally express rat AQP7 and AQP9 [44]. S. cerevisiae cells lacking Fps1 are resistant to As(III) and Sb(III) and accumulate low levels of either metalloid, and complementation with Fps1 reverses this phenotype. When the rat AQP9 gene was expressed in the FPS1 deletion, even higher rates of uptake and even greater sensitivity were observed, suggesting that AQP9 is a better channel for As(III) than Fps1. On the other hand, this may reflect the fact that heterologously expressed AQP9 may not be regulated in yeast. The ability of the four known human members of the aquaglyceroporin family, AQP3, AQP7, AQP9, and AQP10, to facilitate As(III) movement in Xenopus oocytes was also examined [36]. Human AQP9 is a more effective As(III) transporter than AQP7, with much less transport by AQP3 or AQP10. AQP9 is highly expressed in the liver, where it plays an essential role in the uptake of glycerol and urea and in gluconeogenesis [45]. In liver As(III) is methylated to the monomethyl species MAs(III) and subsequently to dimethyl and to some extent trimethyl species [46]. The methylated species are excreted primarily in urine, and the ability to clear arsenic from the body is related to the rate of methylation. How are the methylated species released from liver into blood and eventually to kidney and urine? Rat AQP9 was found to conduct MAs(III) at a higher rate than inorganic As(III) [47]. A cycle of uptake of As(III) and efflux of MAs(III) by AQP9 in liver has been proposed to be a key step in arsenic methylation pathway (Fig. 3). As(III) flows into the hepatocyte down its concentration gradient, where it is glutathionylated and then methylated to MAs(III). Both As(GS)3 and MAs(GS)2 are pumped into bile by multidrug resistance-associated protein 2 (MRP2) [48]. Internally-generated MAs(III) can also flow down its concentration gradient into the bloodstream. AQP9 expression in rat liver was upregulated 20-fold by fasting [45], suggesting that uptake of As(III) and redistribution of MAs(III) may be nutritionally responsive. Mice with an AQP9 disruption are hypersensitive to As(III) and retain arsenic in liver and other organs that express AQP9, suggesting that, like S. meliloti AqpS, the AQP9 is bidirectional and involved in release of arsenic species from liver to blood [49].
Fig. 3. Proposed pathways of metalloid transport in liver.
As(OH)3 in blood flows down its concentration gradient into hepatocytes via AQP9, the major liver isoform. In the cytosol of the hepatocyte, As(OH)3 has two primary fates. After glutathionylation to As(GS)3, it can be pumped into bile by MRP2 or methylated by AS3MT to MAs(OH)2, which then flows down its concentration gradient, also via AQP9, into the blood stream, to bladder and eventually to urine. Thus, bidirectional AQP9 brings As(III) from blood to cytosol and releases the methylated species from cytosol to blood, in both cases passively down a concentration gradient.
Paracelsus, the father of pharmacology, said “the dose makes the poison”. While arsenic is a poison at high concentrations, at lower concentrations arsenic trioxide is an effective chemotherapeutic drug for the treatment of acute promyelocytic leukemia. Although the precise mechanism of action of arsenic trioxide in chemotherapy is not clear; it has been suggested that As2O3 at low concentrations (0.1–0.5 μM) induces differentiation of malignant promyelocytes through inactivation of the PML–RARα fusion protein, while at high concentrations (0.5–2.0 μM), it triggers apoptosis of the promyelocytes and other cancer cells through several different mechanisms [50]. The metalloid also induces proliferation arrest in a number of cancer cells and was tested for the treatment of hematological malignancies and solid tumors, which are mostly refractory to current therapies [50, 51]. To understand the action of arsenical-containing drugs, it is important to elucidate the pathways of drug uptake, factors that modulate the uptake, as well as regulation of drug uptake pathways. Arsenic trioxide almost certainly dissolves to form inorganic As(OH)3, the species that moves through aquaglyceroporin channels [30]. Overexpression of AQP9, AQP7 or AQP3 renders human leukemia cells hypersensitive to the drug as a result of higher steady-state levels of accumulation (R. Mukhopadhyay, unpublished data; [52]). Sensitivity to arsenic trioxide is directly proportional to AQP9 expression in leukemia cells of different lineages [53]. APL cell line NB4 showed the highest expression level of AQP9 and is most sensitive to the drug. In contrast, the chronic myeloid leukemia cell line K562 showed low endogenous AQP9 expression and is insensitive to As2O3. When human AQP9 was overexpressed either in K562 or the promyelocytic leukemia cell line HL60, both became hypersensitive to As(III) and Sb(III) due to higher accumulation of metalloids [52, 53]. Pretreatment of HL60 cells with vitamin D showed higher expression of AQP9 and hypersensitivity to both As(III) and Sb(III). This sensitivity was due to higher rates of uptake of the trivalent metalloids due to increased expression of AQP9 drug uptake system [52]. Pretreatment of HL60 cells with all-trans retinoic acid upregulated AQP9 expression, leading to a significantly increased arsenic uptake [53]. This may explain the improved response from acute promyelocytic leukemia patients when treated concomitantly with all-trans retinoic acid and arsenic trioxide [54, 55]. Thus responsiveness to drug therapy is correlated with increased expression of the drug uptake system. The possibility of using pharmacological agents to increase AQP9 expression delivers the promise of new therapies for the treatment of leukemia.
Conversely, cancer cells might become drug resistant by downregulating aquaglyceroporin expression. When expression of AQPs in an arsenic-resistant cell line (R15) derived from a human lung adenocarcinoma cell line (CL3) was examined, the R15 cells were found to be 10-fold more resistant to As(OH)3 than the parental CL3 cells [56]. R15 cells accumulated less As(OH)3 and expressed little AQP7 or AQP9, but AQP3 mRNA levels were two-fold lower than in CL3 cells. This suggests that, even though AQP3 does not conduct As(III) as well as AQP9 or AQP7, in some cell types it may be responsible for As(III) uptake. When AQP3 expression in CL3 cells was knocked down by RNA interference, the cells exhibited a reduction in As(OH)3 uptake and an increase in resistance. Moreover, overexpression of AQP3 in the human embryonic kidney 293T cells resulted in an increase in both accumulation of and sensitivity to As(OH)3 [56]. Therefore downregulation of aquaglyceroporin expression may lead to metalloid resistance phenotype. In recent years, AQP3 and AQP9 have been found to be upregulated in various malignant tumor types. AQP3 is reported to be expressed in cancers of the brain, lung, kidney, colon, skin, and ovary, and to regulate epidermal growth factor signaling to promote cell proliferation and cell migration in human skin fibroblasts and human ovarian cancer cells. AQP9 overexpression was observed in brain and liver cancer [57]. Again, the conclusion is that increased expression of the drug uptake system can lead to higher drug efficacy.
Interestingly, aquaglyceroporins are not the only proteins capable of transporting As(III) in humans, a discovery first made with yeast. Although disruption of yeast FPS1 leads to loss of As(III) and Sb(III) uptake, yeast grown in media lacking glucose accumulated high levels of As(III), and uptake was inhibited in presence of glucose by approximately 80% [58]. Disruption of FPS1 reduced glucose-independent uptake by only about 25%. The remaining uptake was inhibited by hexoses, including glucose, galactose, mannose and fructose, but not pentoses or disaccharides. A strain lacking FPS1, ACR3 and the 18 genes for hexose permeases (HXT1 to HXT17 and GAL2), exhibited less than 10% of wild type As(III) accumulation. When HXT1, HXT3, HXT4, HXT5, HXT7 or HXT9 were individually expressed in that strain, hexose-inhibitable As(III) uptake was restored. Glucose uptake was reciprocally inhibited by As(III). Thus, while As(III) uptake by Fps1 is important in glucose-containing medium, hexose permeases catalyze the majority of As(III) transport in yeast. The mammalian (both human and rat) glucose transporter GLUT1 was also shown to catalyze transport of both As(III) and CH3As(III) uptake in yeast or in Xenopus oocytes [59, 60]. Thus GLUT1 may be a major pathway uptake of both inorganic and methylated arsenicals in erythrocytes or the epithelial cells of the blood-brain barrier, contributing to arsenic-related cardiovascular problems and neurotoxicity.
3.4 Fish AQPs as metalloid transporters
Fish and shellfish are often exposed to and accumulate high amounts of arsenic, and elevated concentrations of arsenic have been found in the urine of consumers of large amounts of seafood [61]. Other studies have demonstrated arsenic accumulation and biotransformation in various types of fish, which also consume arsenic-containing phyto- and zoöplankton [62]. To date, 18 aquaporins have been reported in fish. Aquaporins in teleosts is divided into three broad sub-groups, the classical aquaporins permeating water, aquaglyceroporins permeating water, glycerol and urea, and unorthodox aquaporins similar to human AQP8 that conduct water and urea but not glycerol. However, zebrafish Aqp8aa and 8ab, but not Aqp8b or gilthead seabrim Aqp8b, are significantly permeable to urea when expressed in Xenopus oocytes [63]. Zebrafish accumulate significant amounts of arsenic in tissues when exposed to As(III) [62]. Five of these zebrafish aquaglyceroporins have been shown to be metalloid transporters: Aqp9a, Aqp9b, Aqp3, Aqp3I and Aqp10. These are most closely related to human AQP9, AQP3 and AQP10, respectively. Each was shown to conduct both water, glycerol and As(III) at similar rates. In contrast, Sb(III) permeation by zebrafish AQP3 and -3I is very poor compared to the other three. On the other hand, MAs(III) uptake by Aqp3I and Aqp10 is 3- to 4-fold higher compared with As(III) [62]. Zebrafish may be a useful model system for arsenic exposure in vertebrates.
3.5 Aquaglyceroporin channels and metalloid uptake in protozoan parasites
The ubiquitous nature of aquaporin channel underlines their importance in life processes. However, most aquaporins are redundant, and individual null mutants, from bacteria to mammals, do not cause lethality [64, 65]. One exception is AQP1 from the parasite protozoan Leishmania (R. Mukhopadhyay and M. Ouellette, unpublished observation). A number of aquaporins have been identified in parasitic protozoa from a single aquaglyceroporin in Plasmodium, to three in Trypanosoma brucei, five in Leishmania, two in Toxoplasma and one in Cryptosporidium [66-69]. A common feature of parasite AQPs is that they are generally better water transporters compared to E. coli (GlpF) [70] and mammalian orthologues. It appears that protozoan aquaglyceroporins are bifunctional and conduct both water and glycerol at reasonable rates [71]. Leishmania AQP1 is involved in accumulation of metalloids in Leishmania promastigotes and amastigotes along with water, glycerol, methylglyoxal, glyceraldehyde and other neutral solutes [67]. The other four AQPs are not involved in metalloid transport (R. Mukhopadhyay, unpublished data) and are closer to classical aquaporins. AQPs are at the interface of host-parasite interactions and could be attractive drug targets and/or mediator of specific drugs such as arsenic and antimony.
3.5.1 Trypanosoma
Trypanosomiasis is an infectious disease of humans and animals of similar etiology and epidemiology. The causative agents of the disease are protozoan parasites of the genus Trypanosoma that live and multiply in their mammalian hosts and are transmitted by the bite of infected insects. T. brucei causes human African trypanosomiasis (HAT), and T. cruzi causes Chagas disease in Latin America. HAT or African sleeping sickness is a painful and long-term illness that is almost always fatal if left untreated. Approximately 300,000 people in Africa are infected with the disease, with 66 million people at risk in the rural areas. One of the most effective drugs is the arsenical melarsoprol (2-(4-(4,6-diamino-1,3,5-triazin-2-ylamino)phenyl)-1,3,2-dithiarsolan-4-yl)methanol). Although melarsoprol is an arsenic-containing drug, it was thought to be too big to pass through AQP channels. Three AQPs have been reported from T. brucei, TbAQP1, TbAQP2 and TbAQP3. All three belong to the aquaglyceroporin category with reasonably good water and glycerol permeability [72]. AQP2 has been recently reported to be involved in susceptibility to melarsoprol in African trypanosomes [73].
3.5.2 Leishmania
Leishmaniasis is a protozoan parasitic infection ranging from self healing cutaneous lesions to non-healing mucocutaneous and visceral ailments caused by Leishmania spp. The disease is endemic in parts of 88 countries across five continents - the majority of the affected countries are in the tropics and subtropics. Approximately 12 million people worldwide are affected by leishmaniasis and 2 million new cases are considered to occur annually. The Leishmania parasite exists in two morphologically distinct forms: promastigotes and amastigotes. The promastigotes reside in the intestinal tract of the sandfly vector and have a slipper like body with an anterior flagellum. Inside the mammalian host, the promastigote forms of the parasites are transformed into amastigotes that appear as small, oval-shaped, aflagelleted structures, and reside in macrophages and other mononuclear phagocytes. The female phlebotomine sandflies are solely responsible for the transmission of Leishmania parasites amongst vertebrate hosts. The disease in humans has been classified in three different forms, each having a broad range of clinical manifestations. Visceral leishmaniasis (VL), the most severe form of the disease, is caused by Leishmania donovani, Leishmania infantum and Leishmania chagasi. Cutaneous leishmaniasis (CL) is caused by a variety of species including Leishmania major, Leishmania tropica, Leishmania mexicana and Leishmania panamensis. Mucocutaneous leishmaniasis (MCL) is caused by Leishmania braziliensis. 90% of MCL cases occur in Bolivia, Brazil and Peru [74]. During operations Desert Storm and Desert Shield in the 1990s, many U.S. soldiers contacted cutaneous leishmaniasis. So many cases of cutaneous infections occurred in the US military between 2003 and 2005 that Lt. Col. Peter Weina, director of Leishmania diagnostics at Walter Reed Army Medical Center commented in Nature Medicine, “This is probably the largest outbreak of leishmaniasis that the US military has ever seen” (February, 2004, Vol:10, page: 110). Additionally, VL is an opportunistic infection among persons who are immunosuppressed, particularly in patients infected with human immunodeficiency virus [75]. The first line compounds against all forms of leishmaniasis are the two pentavalent antimonials, sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime), and clinical drug resistance is common [76, 77]. Leishmania resistant to trivalent antimony has also been reported [78].
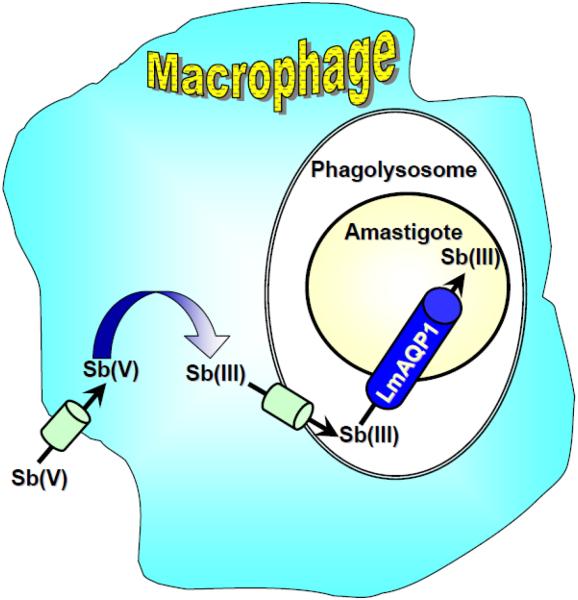
The L. major genome encodes for five aquaporins: LmAQP1, LmAQPα, LmAQPβ, LmAQPγ, and LmAQPδ. While LmAQP1 shows strong similarity to bacterial aquaporins, the other L. major aquaporins (LmAQPα-δ) are closer to plant aquaporins [71]. This is a unique peculiarity of L. major aquaporins since other known parasitic aquaporins are either bacteria-like or plant-like, and not a mixed population [71]. Of the five, only LmAQP1 is involved in uptake of As(III) and Sb(III), the activated form of Pentostam and Glucantime [79] (Fig. 4). The drugs contain Sb(V), and it is postulated that this is reduced to Sb(III) in the cytosol of the macrophage by an as-yet unidentified Sb(V) reductase. Alternatively, it might be taken into the amastigote and reduced to Sb(III) by the parasite reductase LmAcr2 [80]. In either case, Sb(V) has to be transported across the macrophage cytoplasm membrane, and Sb(III) has to penetrate the double membranes of the phagolysosome, and neither of those processes has been identified. It is clear, however, that LmAQP1 is responsible for uptake of Sb(III) into the amastigote, and that mutations in this channel can lead to drug resistance [79]. LmAQP1 is also permeable to water; its water conduction capacity is 65% of that of the classical water channel, human AQP1 [67]. In contrast to Plasmodium and Trypanosome aquaglyceroporins (AQPs) that are inhibited by mercurials, water movement through LmAQP1 is not inhibited by mercuric chloride. LmAQP1 also conducts glycerol, glyceraldehyde, dihydroxyacetone, and sugar alcohols. Expression of LmAQP1 is limited exclusively to the flagellum of promastigotes, while in amastigotes it is found in the flagellar pocket, rudimentary flagellum and contractile vacuoles. LmAQP1 plays an important physiological role in water and solute transport, volume regulation and osmotaxis [67]. Disruption of one of the two LmAQP1 alleles in L. major conferred a 10-fold increase in resistance to Sb(III) [79]. LmAQP1 mRNA levels are significantly less in either the Sb(III)- or As(III)-resistant L. major and Leishmania tarentolae cells, indicating that downregulation of LmAQP1 leads to drug resistance [81]. These findings were corroborated in field isolates from India [82] and Nepal [83]. Therefore, LmAQP1 plays a major role in Leishmania cellular physiology and drug resistance. However, down-regulation of LmAQP1 is not the sole cause of drug resistance in Leishmania. Several other factors include, levels of trypanothione (TSH), antimonite reductase, the MRP homologue PGPA, and an Sb(III)-TSH conjugate exporter in the plasma membrane.
Fig. 4. Model of antimonial drug biotransformation and uptake in macrophage-associated Leishmania amastigotes.
Sb(V) from Pentostam is taken up by macrophages. A portion is reduced to Sb(III), which is transported into the phagolysosome and finally into amastigote via LmAQP1.
Although no crystal structure of LmAQP1 has been determined, structure-function studies have defined the pore mouth residues of LmAQP1 [84]. An extracellular loop near the C-terminus that includes residue Glu152 helps the channel to differentiate between metalloids and glycerol. Ala163 in the same loop is localized near the pore mouth and is critical for channel function; mutations in this residue alter the sensitivity to antimonial drugs [85]. Attempts to generate a null mutation is LmAQP1 have not been successful, suggesting that it may be an essential gene for Leishmania survival and growth (R. Mukhopadhyay and M. Ouellette, unpublished results). Leishmania AQP1 may be a reasonable drug target to control the parasite growth. On one hand, overexpression of this channel makes the parasite hypersensitive to the traditional antimony containing drugs, allowing use of lower concentrations of the drug, which is toxic in high concentrations. It is noteworthy that overexpression of LmAQP1 overcomes every case of drug resistance, laboratory or clinical isolates, no matter what the mechanism of drug resistance [79]. On the other hand, drugs that downregulate expression or inhibit activity of LmAQP1 may be lethal to the parasite. Identification of pharmacological agents that induce AQP1 expression by manipulating the regulatory pathways is a feasible goal. Induction of AQP1 makes the wild type and drug resistant field isolates susceptible to lower concentrations of antimonials. Thus co-administration of inducing agents of AQP1 along with pentavalent antimony will overcome drug resistance and avoid toxicity. However, control of gene expression is complicated in Leishmania. Genes of this parasite are transcribed as a large polycystronic group and regulated at the post transcriptional level largely by the 3’UTR, although controlling cis-elements and trans-acting factors are largely unknown. Mitogen Activated Protein Kinase 2 (MAPK2) was shown to upregulate AQP1 expression, suggesting MAPK2 might be a new drug target [86].
3.6. Plant metalloid channels
Perhaps the greatest surprises have been the large diversity of aquaporins in plants and the discoveries that they are generalized metalloid transporters [87]. Probably due to their need for osmoregulation under multiple environmental stresses, plants have many more aquaporin isoforms than members of other kingdoms, e.g., 33 AQPs are encoded in the rice genome [88]. One subfamily is the nodulin-26–like intrinsic membrane proteins (NIPs). By expression in an FPS1 deletion strain of yeast, NIPs from Arabidopsis thaliana (AtNIP5;1 and AtNIP6;1) Oryza sativa (rice) (OsNIP2;1 and OsNIP3;2), and Lotus japonicus (LjNIP5;1 and LjNIP6;1) were shown to conduct As(III) [89]. This is of concern because rice is a natural arsenic accumulator, which endangers our food supply [90, 91]. In addition, arsenic causes straighthead disease in rice, which is characterized by sterility and can lead to 100% loss of yield.
While uptake of the toxic metalloid arsenic is adventitious, plants have mineral requirements for metalloids not found in animals, in particular for boric (B(III)), and orthosilicic (Si(IV)) acids, which are also taken up by plant aquaglyceroporins (Fig. 1) (for reviews see [18, 87]). While the biochemical mechanisms of boron and silicon utilization by plants is not know, boron deficiency leads to dwarf plants with reduced numbers of spikelets and reduced grain yield, and silicon enhances rice growth and grain yield. Arabidopsis thaliana NIP5;1, a root plasma membrane AQP, is upregulated by boron deficiency, and, when expressed in Xenopus oocytes, NIP5;1 efficiently transports boric acid [92]. Like arsenic and antimony, the metalloid germanium is toxic. By selection for germanium resistance, a rice mutant with reduced silicon uptake was isolated [93]. The ability to use the metalloid Ge for genetic selection indicates that germanic acid is an authentic aquaporin substrate. The gene, termed Lsi1, is an aquaglyceroporin orthologue [94]. It is constitutively expressed in the root and is located in the plasma membrane of the distal sides of cells located near the casparian strips, which controls permeability into the xylem. Lsi1 is on the distal side of the cells. The Lsi2 protein, which is located on the proximal side of the same cells, is a silicon efflux protein [95]. Lsi2 is not an aquaglyceroporin but is distantly related to ArsB, the bacterial arsenite efflux protein [19, 96], suggesting that other arsenic transporters might have broader substrate specificity for metalloids than previously recognized.
It appears, therefore, that NIPs conduct not only arsenite but are generalized metalloid channels [87]. How is that possible? The oxyacids of the metalloids B(OH)3, Si(OH)4, As(OH)3 and Sb(OH)3, are similar in cross-sectional area and have similar distribution of polar and nonpolar faces to glycerol (Fig. 1). Seawater contains approximately 0.4 mM borate and 0.1 mM silicate. Phytoplankton such as diatoms requires silicon for cell wall biosynthesis, so silicon homeostasis may have been a necessity in early evolution. We have hypothesized that the earliest substrates of aquaglyceroporins might have been the required metalloids rather than glycerol or other organic compounds [87].
4. Future perspectives
The identification of aquaglyceroporins as uptake channels for most metalloids provides an understanding of how toxic elements such as arsenic and required elements such as boron and silicon enter the food chain. By engineering their AQPs, food plants such as rice could be made safer by genetically modifying them to exclude arsenic while still accumulating boron and silicon. While this may seem implausible, there is precedence. Leishmania LmAQP1 has an extracellular loop with several glutamate residues. A single E152A mutation reduced uptake of As(III) and Sb(III) to negligible levels without affecting glycerol permeability [84], demonstrating that there is considerable plasticity in the selectivity of the aquaglyceroporin pore. While engineering human genes is less realistic, identification of drugs or pharmacological agents that reduce uptake of As(III) by AQP9 in liver might lead to amelioration of arsenic-related diseases such as bladder cancer. Conversely, pharmacological agents such as vitamin D that act as promoters of uptake of As(III) or Sb(III) could lead to more effective chemotherapeutic treatment of diseases such as acute promyelocytic leukemia or leishmaniasis. In conclusion, the area of metalloid transport is still immature, and new discoveries could lead to improvement in our food supply or more effective treatment of arsenic-related diseases, cancer or parasitic infections.
Highlights.
Aquaporins (AQPs) are metalloid channels ubiquitous in all domains of life.
AQPs conduct boron, germanium, silicon, arsenic, antimony bidirectionally.
As(III), Sb(III) and Ge(IV) are toxic metalloids, and uptake is adventitious.
B(III) and Si(IV) are nontoxic plant growth enhancers under conditions of stress.
Arsenic/antimony compounds are drugs for antimicrobial and anticancer chemotherapy.
Acknowledgements
This work was supported by National Institutes of Health Grant GM55425.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Council NR. Arsenic in Drinking Water: 2001 Update. 2001 http://www.nap.edu/books/0309076293/html/ [PubMed]
- 2.Stone R. Food safety. Arsenic and paddy rice: a neglected cancer risk? Science. 2008;321:184–185. doi: 10.1126/science.321.5886.184. [DOI] [PubMed] [Google Scholar]
- 3.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. J Nutr. 2003;133:1536S–1538S. doi: 10.1093/jn/133.5.1536S. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng WP, Chu HM, How SW, Fong JM, Lin CS, Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968;40:453–463. [PubMed] [Google Scholar]
- 7.Mushak P, Crocetti AF. Risk and revisionism in arsenic cancer risk assessment. Environ Health Perspect. 1995;103:684–689. doi: 10.1289/ehp.95103684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bulletin of the World Health Organization. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 9.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Su HJ, Guo YL, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Christiani DC. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003;14:303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- 11.Putila JJ, Guo NL. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS One. 2011;6:e25886. doi: 10.1371/journal.pone.0025886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- 13.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tofail F, Vahter M, Hamadani JD, Nermell B, Huda SN, Yunus M, Rahman M, Grantham-McGregor SM. Effect of arsenic exposure during pregnancy on infant development at 7 months in rural Matlab, Bangladesh. Environ Health Perspect. 2009;117:288–293. doi: 10.1289/ehp.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JX, Zhou GB, Chen SJ, Chen Z. Arsenic compounds: revived ancient remedies in the fight against human malignancies. Curr Opin Chem Biol. 2012 doi: 10.1016/j.cbpa.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Docampo R, Moreno SN. Current chemotherapy of human African trypanosomiasis. Parasitol Res. 2003;90(Supp 1):S10–13. doi: 10.1007/s00436-002-0752-y. [DOI] [PubMed] [Google Scholar]
- 17.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 18.Zangi R, Filella M. Transport routes of metalloids into and out of the cell: a review of the current knowledge. Chem Biol Interact. 197:47–57. doi: 10.1016/j.cbi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Yang HC, Fu HL, Lin YF, Rosen BP. Pathways of arsenic uptake and efflux. Curr Top Membr. 2012;69:325–358. doi: 10.1016/B978-0-12-394390-3.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg H, Gerdes RG, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131:505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willsky GR, Malamy MH. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol. 1980;144:366–374. doi: 10.1128/jb.144.1.366-374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willsky GR, Malamy MH. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980;144:356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders OI, Rensing C, Kuroda M, Mitra B, Rosen BP. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J Bacteriol. 1997;179:3365–3367. doi: 10.1128/jb.179.10.3365-3367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng YL, Liu Z, Rosen BP. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J Biol Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- 25.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nature reviews. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 26.Zardoya R. Phylogeny and evolution of the major intrinsic protein family. Biology of the cell / under the auspices of the European Cell Biology Organization. 2005;97:397–414. doi: 10.1042/BC20040134. [DOI] [PubMed] [Google Scholar]
- 27.Agre P. Nobel Lecture. Aquaporin water channels. Biosci Rep. 2004;24:127–163. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- 28.Danielson JA, Johanson U. Phylogeny of major intrinsic proteins. Advances in experimental medicine and biology. 679:19–31. doi: 10.1007/978-1-4419-6315-4_2. [DOI] [PubMed] [Google Scholar]
- 29.Pao GM, Wu LF, Johnson KD, Hofte H, Chrispeels MJ, Sweet G, Sandal NN, Saier MH., Jr. Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991;5:33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramírez-Solis A, Mukopadhyay R, Rosen BP, Stemmler TL. Experimental and theoretical characterization of arsenite in water: insights into the coordination environment of As-O. Inorg Chem. 2004;43:2954–2959. doi: 10.1021/ic0351592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porquet A, Filella M. Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chemical research in toxicology. 2007;20:1269–1276. doi: 10.1021/tx700110m. [DOI] [PubMed] [Google Scholar]
- 32.Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H. Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti. J Bacteriol. 2005;187:6991–6997. doi: 10.1128/JB.187.20.6991-6997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu B, Song J, Beitz E. Novel channel enzyme fusion proteins confer arsenate resistance. J Biol Chem. 2010;285:40081–40087. doi: 10.1074/jbc.M110.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wysocki R, Chery CC, Wawrzycka D, Van Hulle M, Cornelis R, Thevelein JM, Tamás MJ. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol Microbiol. 2001;40:1391–1401. doi: 10.1046/j.1365-2958.2001.02485.x. [DOI] [PubMed] [Google Scholar]
- 35.Nozawa A, Takano J, Kobayashi M, von Wiren N, Fujiwara T. Roles of BOR1, DUR3, and FPS1 in boron transport and tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2006;262:216–222. doi: 10.1111/j.1574-6968.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Carbrey JM, Agre P, Rosen BP. Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochem Biophys Res Commun. 2004;316:1178–1185. doi: 10.1016/j.bbrc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Tamás MJ, Luyten K, Sutherland FC, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J, Gustafsson L, Thevelein JM, Hohmann S. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol. 1999;31:1087–1104. doi: 10.1046/j.1365-2958.1999.01248.x. [DOI] [PubMed] [Google Scholar]
- 38.Thorsen M, Di Y, Tangemo C, Morillas M, Ahmadpour D, Van der Does C, Wagner A, Johansson E, Boman J, Posas F, Wysocki R, Tamas MJ. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Molecular biology of the cell. 2006;17:4400–4410. doi: 10.1091/mbc.E06-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beese SE, Negishi T, Levin DE. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet. 2009;5:e1000738. doi: 10.1371/journal.pgen.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maciaszczyk-Dziubinska E, Wawrzycka D, Sloma E, Migocka M, Wysocki R. The yeast permease Acr3p is a dual arsenite and antimonite plasma membrane transporter. Biochimica et biophysica acta. 2012;1798:2170–2175. doi: 10.1016/j.bbamem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Nat. Acad Sci USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci U S A. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Styblo M, Rosen BP. Methylarsonous acid transport by aquaglyceroporins. Environ Health Perspect. 2006;114:527–531. doi: 10.1289/ehp.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leslie EM. Arsenic-glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs) J Inorg Biochem. 2011;108:141–149. doi: 10.1016/j.jinorgbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Carbrey JM, Song L, Zhou Y, Yoshinaga M, Rojek A, Wang Y, Liu Y, H.L. L, DiCarlo SE, Nielsen S, Rosen BP, Agre P, Mukhopadhyay R. Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc Natl Acad Sci U S A. 2009;106:15956–15960. doi: 10.1073/pnas.0908108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33:542–564. doi: 10.1016/j.ctrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Verstovsek S, Giles F, Quintas-Cardama A, Perez N, Ravandi-Kashani F, Beran M, Freireich E, Kantarjian H. Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol Oncol. 2006;24:181–188. doi: 10.1002/hon.787. [DOI] [PubMed] [Google Scholar]
- 52.Bhattacharjee H, Carbrey J, Rosen BP, Mukhopadhyay R. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun. 2004;322:836–841. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 54.Aribi A, Kantarjian HM, Estey EH, Koller CA, Thomas DA, Kornblau SM, Faderl SH, Laddie NM, Garcia-Manero G, Cortes JE. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109:1355–1359. doi: 10.1002/cncr.22524. [DOI] [PubMed] [Google Scholar]
- 55.Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philosophical transactions of the Royal Society of London. 2007;362:959–971. doi: 10.1098/rstb.2007.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee TC, Ho IC, Lu WJ, Huang JD. Enhanced expression of multidrug resistance-associated protein 2 and reduced expression of aquaglyceroporin 3 in an arsenic-resistant human cell line. J Biol Chem. 2006;281:18401–18407. doi: 10.1074/jbc.M601266200. [DOI] [PubMed] [Google Scholar]
- 57.Saito Y, Furukawa T, Obata T, Saga T. Molecular imaging of aquaglycero-aquaporins: its potential for cancer characterization. Biol Pharm Bull. 36:1292–1298. doi: 10.1248/bpb.b13-00079. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z, Boles E, Rosen BP. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J Biol Chem. 2004;279:17312–17318. doi: 10.1074/jbc.M314006200. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang X, McDermott JR, Ajees AA, Rosen BP, Liu Z. Trivalent arsenicals and glucose use different translocation pathways in mammalian GLUT1. Metallomics. 2010;2:211–219. doi: 10.1039/b920471g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gueguen M, Amiard JC, Arnich N, Badot PM, Claisse D, Guerin T, Vernoux JP. Shellfish and residual chemical contaminants: hazards, monitoring, and health risk assessment along French coasts. Rev Environ Contam Toxicol. 213:55–111. doi: 10.1007/978-1-4419-9860-6_3. [DOI] [PubMed] [Google Scholar]
- 62.Hamdi M, Sanchez MA, Beene LC, Liu Q, Landfear SM, Rosen BP, Liu Z. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol Biol. 2009;10:104. doi: 10.1186/1471-2199-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerda J, Finn RN. Piscine aquaporins: an overview of recent advances. J Exp Zool A Ecol Genet Physiol. 313:623–650. doi: 10.1002/jez.634. [DOI] [PubMed] [Google Scholar]
- 64.Mukhopadhyay R, Beitz E. Metalloid transport by aquaglyceroporins: consequences in the treatment of human diseases. Adv Exp Med Biol. 679:57–69. doi: 10.1007/978-1-4419-6315-4_5. [DOI] [PubMed] [Google Scholar]
- 65.Fadiel A, Isokpehi RD, Stambouli N, Hamza A, Benammar-Elgaaied A, Scalise TJ. Protozoan parasite aquaporins. Expert Rev Proteomics. 2009;6:199–211. doi: 10.1586/epr.09.10. [DOI] [PubMed] [Google Scholar]
- 66.Beitz E. Aquaporin water and solute channels from malaria parasites and other pathogenic protozoa. ChemMedChem. 2006;1:587–592. doi: 10.1002/cmdc.200500105. [DOI] [PubMed] [Google Scholar]
- 67.Figarella K, Uzcategui NL, Zhou Y, Lefurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 68.Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- 69.Chen XM, O'Hara SP, Huang BQ, Splinter PL, Nelson JB, LaRusso NF. Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc Natl Acad Sci U S A. 2005;102:6338–6343. doi: 10.1073/pnas.0408563102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song J, Almasalmeh A, Krenc D, Beitz E. Molar concentrations of sorbitol and polyethylene glycol inhibit the Plasmodium aquaglyceroporin but not that of E. coli: involvement of the channel vestibules. Biochimica et biophysica acta. 1818:1218–1224. doi: 10.1016/j.bbamem.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 71.Beitz E. Aquaporins from pathogenic protozoan parasites: structure, function and potential for chemotherapy. Biol Cell. 2005;97:373–383. doi: 10.1042/BC20040095. [DOI] [PubMed] [Google Scholar]
- 72.Uzcategui NL, Szallies A, Pavlovic-Djuranovic S, Palmada M, Figarella K, Boehmer C, Lang F, Beitz E, Duszenko M. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J Biol Chem. 2004;279:42669–42676. doi: 10.1074/jbc.M404518200. [DOI] [PubMed] [Google Scholar]
- 73.Baker N, Glover L, Munday JC, Aguinaga Andres D, Barrett MP, de Koning HP, Horn D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhattacharjee H, Mukhopadhyay R. Drug resistance in Leishmania. In: Lerner M.O.a.S., editor. Antimicrobial drug resistance: Principles and Practice for the Clinic and Bench. Humana Press; 2009. [Google Scholar]
- 75.Choi CM, Lerner EA. Leishmaniasis: recognition and management with a focus on the immunocompromised patient. Am J Clin Dermatol. 2002;3:91–105. doi: 10.2165/00128071-200203020-00003. [DOI] [PubMed] [Google Scholar]
- 76.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugere B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson JE, Tally JD, Ellis WY, Mebrahtu YB, Lawyer PG, Were JB, Reed SG, Panisko DM, Limmer BL. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania ssp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990;43:464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- 78.Ouellette M, Haimeur A, Grondin K, Legare D, Papadopoulou B. Amplification of ABC transporter gene pgpA and of other heavy metal resistance genes in Leishmania tarentolae and their study by gene transfection and gene disruption. Methods Enzymol. 1998;292:182–193. doi: 10.1016/s0076-6879(98)92015-8. [DOI] [PubMed] [Google Scholar]
- 79.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Messier N, Ouellette M, Rosen BP. R. Mukhopadhyay, Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug Pentostam. J Biol Chem. 2004;279:37445–27451. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]
- 81.Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Molecular Microbiology. 2005;57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- 82.Mandal S, Maharjan M, Singh S, Chatterjee M, Madhubala R. Assessing aquaglyceroporin gene status and expression profile in antimony-susceptible and - resistant clinical isolates of Leishmania donovani from India. J Antimicrob Chemother. doi: 10.1093/jac/dkp468. [DOI] [PubMed] [Google Scholar]
- 83.Decuypere S, Rijal S, Yardley V, De Doncker S, Laurent T, Khanal B, Chappuis F, Dujardin JC. Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob Agents Chemother. 2005;49:4616–4621. doi: 10.1128/AAC.49.11.4616-4621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uzcategui NL, Zhou Y, Figarella K, Ye J, Mukhopadhyay R, Bhattacharjee H. Alteration in glycerol and metalloid permeability by a single mutation in the extracellular C-loop of Leishmania major aquaglyceroporin LmAQP1. Mol Microbiol. 2008;70:1477–1486. doi: 10.1111/j.1365-2958.2008.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukhopadhyay R, Mandal G, Atluri VS, Figarella K, Uzcategui NL, Zhou Y, Beitz E, Ajees AA, Bhattacharjee H. The role of alanine 163 in solute permeability of Leishmania major aquaglyceroporin LmAQP1. Mol Biochem Parasitol. 175:83–90. doi: 10.1016/j.molbiopara.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandal G, Sharma M, Kruse M, Sander-Juelch C, Munro LA, Wang Y, Vilg JV, Tamas MJ, Bhattacharjee H, Wiese M, Mukhopadhyay R. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol. 85:1204–1218. doi: 10.1111/j.1365-2958.2012.08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhattacharjee H, Mukhopadhyay R, Thiyagarajan S, Rosen BP. Aquaglyceroporins: ancient channels for metalloids. J Biol. 2008;7:33. doi: 10.1186/jbiol91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 89.Bienert GP, Thorsen M, Schussler MD, Nilsson HR, Wagner A, Tamas MJ, Jahn TP. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC biology. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003-2004 NHANES Data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 92.Takano J, Miwa K, Fujiwara T. Boron transport mechanisms: collaboration of channels and transporters. Trends in plant science. 2008;13:451–457. doi: 10.1016/j.tplants.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Ma JF, Tamai K, Ichii M, Wu GF. A rice mutant defective in Si uptake. Plant Physiol. 2002;130:2111–2117. doi: 10.1104/pp.010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 95.Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature. 2007;448:209–212. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 96.Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]