Highlights
-
•
Loss aversion is present in adolescents.
-
•
Levels of loss aversion are not modulated by clinical anxiety in adolescents.
-
•
The 5HTT gene modulates levels of loss aversion in clinically anxious patients.
-
•
A subset of anxious adolescents, high 5HTT-expressers, has low lambda and high impulsivity.
-
•
High 5HTT-expression may expose anxious patients to comorbid externalizing disorders.
Keywords: Risk-taking, Microeconomics, Lambda, Impulsivity, Development, Risk-avoidance
Abstract
Loss aversion, a well-documented behavioral phenomenon, characterizes decisions under risk in adult populations. As such, loss aversion may provide a reliable measure of risky behavior. Surprisingly, little is known about loss aversion in adolescents, a group who manifests risk-taking behavior, or in anxiety disorders, which are associated with risk-avoidance. Finally, loss aversion is expected to be modulated by genotype, particularly the serotonin transporter (SERT) gene variant, based on its role in anxiety and impulsivity. This genetic modulation may also differ between anxious and healthy adolescents, given their distinct propensities for risk taking. The present work examines the modulation of loss aversion, an index of risk-taking, and reaction-time to decision, an index of impulsivity, by the serotonin-transporter-gene-linked polymorphisms (5HTTLPR) in healthy and clinically anxious adolescents. Findings show that loss aversion (1) does manifest in adolescents, (2) does not differ between healthy and clinically anxious participants, and (3), when stratified by SERT genotype, identifies a subset of anxious adolescents who are high SERT-expressers, and show excessively low loss-aversion and high impulsivity. This last finding may serve as preliminary evidence for 5HTTLPR as a risk factor for the development of comorbid disorders associated with risk-taking and impulsivity in clinically anxious adolescents.
1. Introduction
Loss aversion is a classical concept in behavioral economics (Kahneman and Tversky, 1979). Indeed, a prominent theoretical account of economic decision-making, Prospect Theory (Kahneman and Tversky, 1979), indicates that people are more sensitive to losses than gains, typically by a factor of two (Novemsky and Kahneman, 2005). Loss aversion is traditionally measured using a series of mixed gambles that vary in magnitude of potential gains and losses. It is computed as the ratio (lambda) of the contribution of loss magnitude to that of gain magnitude to the subject's decision (e.g., Tom et al., 2007). This mathematically modeled, extensively studied behavioral characteristic appears highly reliable in adults. It captures an aspect of risk-avoidance and, its converse, risk-taking, both, of which are particularly relevant to adolescence and anxiety.
Adolescence is a unique time in life, when developmental factors might have opposing effects on loss aversion. On the one hand, adolescents experience a peak in their risk-taking behavior (Spear, 2000), which, at face value, could be associated with low levels of loss aversion (Kahneman and Lovallo, 1993). Thus, adolescence might be expected to signal low levels of loss aversion. On the other hand, adolescence also is a time when anxiety increases, and prior research links anxiety to risk avoidance (Lorian and Grisham, 2010, Maner et al., 2007, Mueller et al., 2010), which could be associated with high levels of loss aversion. Taken together, these two sets of contrasting findings raise major questions on the way in which loss aversion manifests in adolescents, in general, as well as in adolescents with high levels of anxiety. At present, only one study has compared loss aversion in adolescents and adults, and this study failed to detect differences in the measure of loss aversion, lambda, between these age groups (Barkley-Levenson et al., 2013). No studies have yet assessed the relationship between loss aversion and anxiety at any age.
Finally, anxiety is a heterogeneous set of clinical conditions, showing variable relationships to environmental risks, such as stress or trauma, and genetic factors. Particular interest has arisen concerning the relationship between anxiety and variation in the serotonin transporter (SERT) gene (Bengel et al., 1999, Gonda et al., 2009, Lesch et al., 1996, Sen et al., 2004). Understanding the contribution of genotype to anxiety is important because genetics may moderate relationships between anxiety and its neurobiological correlates (e.g., Pine et al., 2010, Xu et al., 2006). Accordingly, SERT variants could also moderate the relation of anxiety with loss aversion, a relation which, in the future, could be captured at the neural level in follow-up studies using functional neuroimaging tools.
This study tests four hypotheses. We expect that (1) adolescents would exhibit some degree of loss aversion, since loss aversion is a well-established phenomenon across adult populations (Novemsky and Kahneman, 2005); (2) Loss aversion would be higher in clinically anxious compared to healthy adolescents; (3) Loss aversion would be influenced by SERT gene variants. Specifically, we expect that high-expressers (LaLa carriers) would show lower levels of loss aversion based on the role of this gene variant in impulsive-related behaviors (e.g., Beitchman et al., 2003, Curran et al., 2005, Manor et al., 2001, Retz et al., 2008, Retz et al., 2002, Seeger et al., 2001, Zoroglu et al., 2002), relative to low-expressers (S/Lg carriers) who would manifest higher levels of loss aversion based on the role of this gene variant in anxiety and harm avoidance (e.g., Bengel et al., 1999, Gonda et al., 2009, Lesch et al., 1996, Sen et al., 2004). Similarly, we anticipate differences in reaction time to execute a decision involving risky options, such that fast reaction time, indicative of impulsivity, would characterize low loss-averse individuals, whereas long reaction time would characterize high loss-averse individuals. Finally, (4) we expect that genotype would moderate the relationship between anxiety and loss aversion (lambda), emerging in an interaction between diagnosis and genotype.
2. Methods
2.1. Participants
A total of 66 Caucasian adolescents, 27 with an anxiety disorder and 39 healthy comparisons were assessed on the Loss Aversion task, a paradigm of mixed monetary gambles (Tom et al., 2007). Most patients carried more than one anxiety disorder, as delineated in Table 1. In addition, five patients met another non-anxiety comorbid diagnosis (see Table 1).
Table 1.
Distribution of diagnoses (a) in the anxious adolescents as a whole and (b) by genotype. As expected based on comorbid anxiety disorders, the total numbers of diagnoses exceed the size of each sample. For example, the high-expresser sample includes 9 subjects, while the total number of diagnoses amount to 11. Two high-expresser subjects had 2 anxiety diagnoses. GAD, Generalized Anxiety Disorder; SocPh, Social Phobia; SAD, Separation Anxiety Disorder.
| (a) | |||
|---|---|---|---|
| Total | Comorbidity | ||
| GAD | 16 | GAD-only | 12 |
| GAD/SAD/SocPh | 2 | ||
| GAD/SAD | 2 | ||
| SocPh | 7 | SocPh-only | 4 |
| Soc-Ph/SAD | 1 | ||
| SAD | 11 | SAD-only | 6 |
| (b) | |||
|---|---|---|---|
| Patients | GAD | SocPh | SAD |
| High expressers (n = 9) | 4 (44.4%) | 1 (11.1%) | 6 (66.7%) |
| Low expressers (n = 18) | 12 (66.7%) | 6 (33.3%) | 5 (27.8%) |
Participants were recruited through local newspaper advertisements and word of mouth, and the study was approved by the National Institute of Mental Health Institutional Review Board. The group of anxious adolescents was recruited for a treatment study of anxiety, and the comparisons were recruited from the same community. For the participants enrolled in the treatment study of anxiety, the task was completed during an initial research period prior to treatment entry. The parents of all children gave informed consent, and minors gave informed assent.
Inclusion criteria for comparisons comprised (1) age between 8 and 17 years; (2) absence of acute or chronic medical problems; and (3) absence of current or past psychiatric disorders. Inclusion criteria for anxious youths included: (1) primary diagnosis of an anxiety disorder based on a semi-structured diagnostic interview (K-SADS; Kaufman et al., 1997) completed by a trained clinical psychologist; (2) desire for outpatient treatment; (3) age between 8 and 17 years. Exclusion criteria for all participants consisted of (1) current use of any psychoactive substance; (2) current Tourette's syndrome, obsessive-compulsive disorder, PTSD, conduct disorder, exposure to extreme trauma, or suicidal ideation; (3) lifetime history of mania, psychosis, or pervasive developmental disorder; or (4) IQ < 70.
2.2. Assessment tools and genotyping
IQ was measured using the vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence (WASI; Weschler, 1999). Socioeconomic status was obtained through parental report and calculated based on Hollingshead's index of social position for education and Hollingshead's categories for employment (Hollingshead, 1975). The parent and child version of the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997, Muris et al., 1999) were collected, and the average scores for parent and child SCARED were used as the index of anxiety severity (SCAREDpc).
DNA extraction and genotyping for this sample was conducted according to published procedures (Hu et al., 2006). Genomic DNA was prepared from laboratory-collected saliva samples using Oragene DNA kits (DNA genotek, Ottowa, Ontario, Canada), or, when available, blood samples. The SLC6A4 promoter (5HTTLPR) genotyping was performed in two stages using size discrimination for the S (103 bp), L (146 bp), La (146 bp) and Lg (61 bp) alleles. The 5HTTLPR region was amplified in a 20 μl reaction: 1× Optimized Buffer A, 1× PCR enhancer, 0.25 μM each primer [FAM-ATCGCTCCTGCATCCCCCATTAT (forward), GAGGTGCAGGGGGATGCTGGAA (reverse)], 0.125 μM dNTP, 10 ng DNA, 1.25μ Platinum Taq polymerase (all Invitrogen Corp); 95 °C (5 min), 40 cycles of 94 °C (30 s), 52 °C (30 s), 68 °C (1 min), and a final elongation, 68 °C (10 min). S and L genotypes were discriminated directly from the PCR reaction products, rs25531 genotype was determined by digesting 10 μl PCR mix with 100 units MspI (37 °C, 1 h, 1× restriction buffer). Samples were mixed with deionized formamide and GeneScan™-500 ROX Size Standard (Applied Biosystems), and genotypes resolved on a 3730 DNA Analyzer (Applied Biosystems).
Variation at the closely linked SNP rs25531 also modulates SLC6A4 expression, with the relatively common Lg allele having a similar expression profile to the S allele (Hu et al., 2006). As such, participants were assigned to one of two groups based on the presumed neurotransmitter expression level. For the current study, “high-expressers” will refer to those with the LaLa-allele and “low-expressers” will refer to those with SLa, SLg, and SS-alleles (S/Lg). In this sample 21 (9 anxious and 12 healthy) subjects were high-expressers and 45 (18 anxious and 27 healthy) were low-expressers.
2.3. Task description
Participants completed a computerized betting task in which they were asked to either take or reject a 50/50 chance to win one amount of money or lose another amount of money, similar to a coin toss (Tom et al., 2007). Based on the notion that losses loom larger than gains (Tversky and Kahneman, 1992), gains were scaled between $10 and $40 by increments of $2, and losses were scaled between $5 and $20 by increments of $1. All combinations between potential gain and potential loss values were presented in a random order (256 total gambles).
In order to assess individual differences in the level of attractiveness of each bet, as well as to discourage participants from establishing a set response pattern while playing, 4 response options were offered to the participants. Participants could choose to (1) strongly take, (2) weakly take, (3) strongly pass or (4) weakly pass on each of the gambles presented. Feedback was not provided and the task was self-paced. Participants were told that at the completion of the game, three randomly chosen gambles would determine their compensation or loss. For each bet they chose to “take” whilst playing the game, they were told that an actual coin toss would establish if they won or lost money. If they chose to “pass” on the bets, no money would be added or subtracted from their total compensation. At the end of the task, subjects were told the cumulative amount of money they would have won or lost from 3 random bets.
2.4. Statistical analyses
We computed a behavioral index of loss aversion (lambda) by fitting a logistic regression to each participant's responses collected during the task with sizes of gain and loss used as independent variables. Lambda was calculated as the ratio of the absolute loss response to the gain response (Tom et al., 2007).
Reaction time (RT) was calculated on valid trials. Invalid RT was defined as RT < 150 ms or >2.5 SDs above the mean (e.g., impulsive responses or initial inattention to the cue). There was an average of 5.7 (SD = 1.9) outliers per subject (out of 256 trials) across the whole sample, and the average number of outliers did not differ across genotype groups (F = 3.0, df = 1,61, p = 0.08) or diagnostic groups (F = 0.97, df = 1,61, p = 0.39). The range was within what could be expected for executing a decision on a guessing task. Decision-making tasks usually allow 3000 ms for executing a selection between numerical options (e.g., Tom et al., 2007, Barkley-Levenson et al., 2013, Eshel et al., 2007).
Given the differences in sample size, we used the Levene's test to test for homogeneity of lambda variance across groups. Variance did not differ between diagnostic groups (F = 0.54, df = 1,64, p = 0.47) or genotype groups (F = 1.82, df = 1,64, p = 0.19). Therefore, no correction procedure was necessary.
We used a 2-way ANOVA to examine the effects and interaction of Diagnosis (anxious, comparison) and Genotype (high-expressers, low-expressers) on demographic variables to identify potential confounders. The only potential confounder was age, which was correlated with lambda (see results). Therefore, all analyses were controlled for age. However, for completeness, we also show the results without controlling for age. Lambda (measure of loss aversion) and reaction time (RT) to bet or pass were analyzed using 2-way ANCOVAs, with Diagnosis and Genotype as the between-subjects factors, and age as the covariate of nuisance.
In addition, analyses were also conducted after correcting not only for age but also for differences in RT. Finally, anxiety scores (SCAREDpc) were also used as complementary analyses to examine the effects of anxiety as a continuous variable instead of using the categorical variable of Diagnosis.
3. Results
3.1. Demographics
Table 2 summarizes the description of the demographic variables as a function of Diagnosis and Genotype. The only demographic that differed between groups was age. The anxiety patients (M = 11.50, SD = 2.53) were younger than the healthy adolescents (M = 13.10, SD = 2.54). There were no age differences as a function of genotype status.
Table 2.
Demographic and clinical characteristics of the sample by Diagnosis and Genotype (A-LaLa, anxious high-expressers; A-S/Lg, anxious low-expressers; H-LaLa, healthy high-expressers; H-S/Lg, healthy low-expressers).
| Statistics (p values) |
|||||||
|---|---|---|---|---|---|---|---|
| A-LaLa | A-S/Lg | H-LaLa | H/S/Lg | Diagnosis | Genotype | Diag.*Genotype | |
| N | 9 | 18 | 12 | 27 | |||
| Male/female | 4/5 | 8/10 | 5/7 | 14/13 | NS | NS | NS |
| Age mean (SD) | 11.0 (2.4) | 11.7 (2.6) | 14.3 (2.7) | 12.6 (2.3) | 0.003 | NS | NS |
| IQ mean (SD) | 115.1 (12.3) | 114.4 (11.8) | 108.8 (11.5) | 117.9 (10.7) | NS | NS | NS |
| SES mean (SD) | 37.6 (17.7) | 29.5 (9.4) | 35.2 (9.8) | 34.5 (14.8) | NS | NS | NS |
| Comorbid non-anxiety Diagnoses | MDD n = 1 ADHD n = 1 |
ADHD n = 1 ODD n = 2 |
|||||
| SCAREDpc mean (SD) | 30.6 (19.6) | 29.9 (5.9) | 7.5 (7.1) | 6.7 (6.0) | <0.0001 | NS | NS |
As expected, the SCAREDpc scores were significantly higher in the anxious than the healthy group.
3.2. Lambda (loss aversion)
Lambda across the whole group was positive with an average of 1.39 (SD = 0.80) which was significantly different from zero (p < 0.0001). Lambda was not modulated by gender, IQ, SES or SCAREDpc across the whole sample.
However, lambda was positively correlated with age in the whole group (r = 0.37, F = 4.32, df = 1,63, p = 0.04). This correlation remained significant after controlling for Diagnosis or Genotype (F = 4.49, df = 1.62, p = 0.019). As age increased across the sample, loss aversion also increased.
We examined whether the effect of age on lambda was moderated by Genotype or Diagnosis. Regarding Genotype, lambda was modulated differently by age in high-expressers vs. low-expressers (age*genotype: F = 4.98, df = 1,64, p = 0.029). Lambda increased with age in the high expressers (LaLa: r = 0.63, p = 0.002, n = 21), but was not influenced by age in the low-expressers (S/Lg: r = 0.04, p = 0.81, n = 45). As a reminder, the age distribution was similar in both genotype groups (t(64) = 0.95, p = 0.345). Regarding Diagnosis, diagnostic group showed a trend effect on the relationship of lambda with age (age*Diagnosis: F = 0.3, df = 1,64, p = 0.09). Lambda increased with age in both diagnostic groups, significantly so in healthy adolescents (F = 6.03, df = 1,37, p = 0.019), and not significantly so in anxious adolescents (F = 0.82, df = 1,25, p = 0.37).
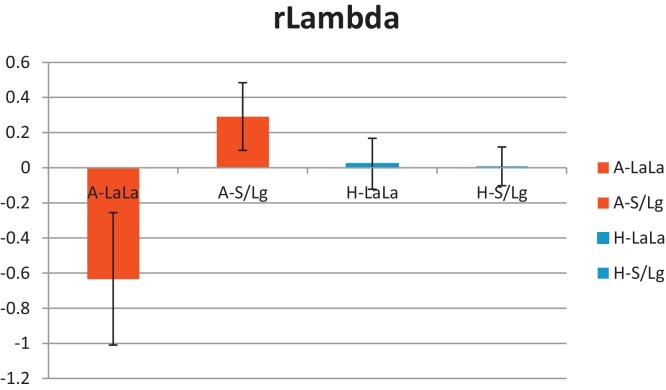
A two-way ANCOVA tested main effects and interactions of Diagnosis and Genotype on lambda with age as the nuisance covariate. Results revealed no main effect of Diagnosis (F = 1.01, df = 1,61, p = 0.32), indicating that clinically anxious adolescents did not differ from healthy adolescents on lambda (anxious: mean = 1.30, SD = 1.03; healthy: mean = 1.47, SD = 0.59). However, the Diagnosis by Genotype interaction was statistically significant, F (1,61) = 5.78, p = .019 (see Fig. 1). Genotype also showed a main effect on lambda (F = 5.2, df = 1,61, p = 0.027). This effect indicated that lambda was lower in high-expressers (LaLa-allele: lambda lsmeans 1.09) than low-expressers (S/Lg-allele: lambda lsmeans 1.54).
Fig. 1.
Mean (standard error) of lambda (residuals after age correction, r-lambda) in the anxious and healthy groups by genotype (A-LaLa, anxious high-expressers; A-S/Lg, anxious low-expressers; H-LaLa, healthy high-expressers; H-S/Lg, healthy low-expressers).
Findings were similar when not correcting for age (two-way ANOVA): Genotype had a main effect on lambda (F = 4.24, df = 1,62, p = 0.04) characterized by lower lambda in high-expressers (LaLa-Lambda, mean = 1.2, SD = 1.0) than low-expressers (S/Lg-lambda mean = 1.5, SD = 0.66). In addition, Diagnosis by Genotype interaction on lambda was highly significant (F = 8.14, df = 1,62, p = 0.006), and there was a trend for Diagnosis to influence lambda (anxious: lambda mean = 1.3 SD = 1.02; healthy: lambda mean = 1.5, SD = 0.59; F = 3.15, df = 1,62, p = 0.08).
The two-way ANCOVA was decomposed into 2 one-way ANCOVAs to examine the effect of Genotype on lambda in the anxiety group and the healthy group separately. Genotype had a significant effect on lambda in the anxious group (F = 5.96, df = 1, 24, p = 0.02). Anxious adolescents who were high-expressers (LaLa, M = 0.64, SD = 1.26) had lower lambda scores than anxious adolescents who were low-expressers (S/Lg, M = 1.62, SD = .73). Healthy children did not differ on lambda scores by Genotype (high-expressers: M = 1.57, SD = .54; low-expressers: M = 1.41, SD = .61, F = 0.0, df = 1,36, p = 0.96; see Fig. 1).
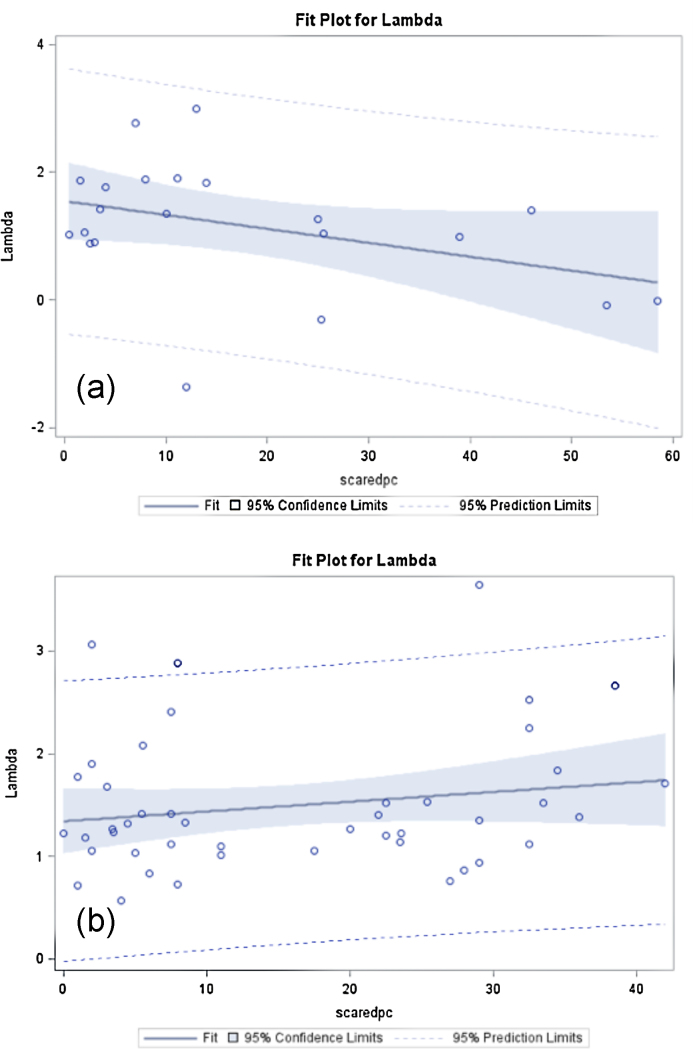
Complementary analyses were run using anxiety severity (SCAREDpc scores) as a continuous variable instead of the categorical variable Diagnosis. The correlations between lambda and SCAREDpc differed significantly between genotypes (F = 4.2, df = 1,61, p = 0.045). The high-expresser group showed a negative correlation between lambda and SCAREDpc (r = −0.39, p = 0.08, n = 21) (Fig. 2a), whereas the low-expresser showed a non-significant positive association (r = 0.18, p = 0.22, n = 45) (Fig. 2b).
Fig. 2.
Correlation plots between lambda and SCAREDpc by genotype: (a) high-expressers (LaLa) show a negative correlation between anxiety severity and lambda: the more anxious, the less loss-averse individuals tended to be if they were high expressers; and (b) low-expressers (S/Lg) show a positive non-significant correlation between anxiety severity and lambda.
3.3. Reaction time (RT)
Mean (SD) RTs are listed in Table 3. Across the whole sample, RT was shorter (F = 19.6 df = 1,61, p < .001) for bet-taking (mean = 1800.8 ms, SD = 470.5) than bet-rejecting (mean = 2098.9 ms, SD = 543.1). Since this pattern was similar among the genotype groups as well as among the diagnostic groups, type of decision (bet, pass) was not used as a factor in the subsequent analyses.
Table 3.
Mean (SD) reaction time by Genotype and Diagnosis (A-LaLa, anxious high-expressers; A-S/Lg, anxious low-expressers; H-LaLa, healthy high-expressers; H-S/Lg, healthy low-expressers).
| A-LaLa | A-S/Lg | H-LaLa | H-S/Lg | |
|---|---|---|---|---|
| N | 9 | 18 | 12 | 27 |
| Mean (ms) | 1573.3 | 2020.3 | 1812.2 | 1876.8 |
| SD | 515.519 | 525.417 | 491.116 | 394.987 |
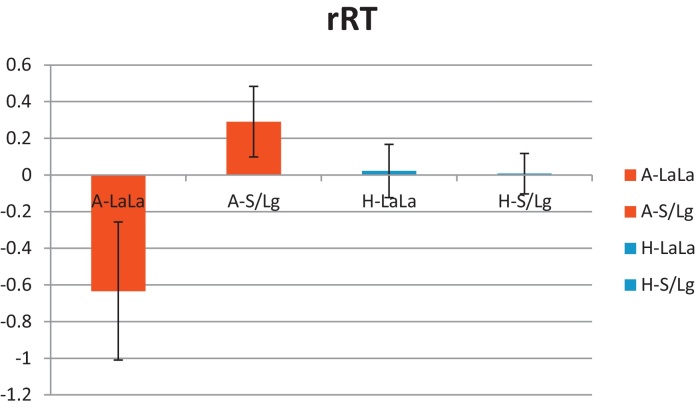
A 2-way ANCOVA with age as a nuisance covariate was used to examine the effects of Diagnosis and Genotype on RT. The interaction of Diagnosis by Genotype was significant (F = 4.82, df = 1,61, p = 0.03). This interaction reflected shorter RT in low- than high-expressers in the patient group, and no RT difference by genotype in the healthy group (RT lsmeans in ms: anxious-LaLa = 1502.6, anxious-S/Lg = 1985.5, healthy-LaLa = 1904.4, healthy-S/Lg = 1882.7) (see Fig. 3). There was a trend for a main effect of Genotype (F = 2.8, df = 1,61, p = 0.09). The main effect of Diagnosis was not significant (F = 1.60, df = 1,61, p = 0.21).
Fig. 3.
Mean (standard error) of reaction time (residuals after age correction, rRT) in the anxious and healthy groups by genotype (A-LaLa: anxious high-expressers; A-S/Lg: anxious low-expressers; H-LaLa: healthy high-expressers; H-S/Lg: healthy low-expressers).
Uncorrected for age, the main effect of Genotype became significant (F = 4.18, df = 1,62, p = 0.045),with RT being shorter in high-expressers (LaLa, RT mean = 1709.8 ms, SD = 503.6) than low-expressers (S/Lg, RT mean = 1934.2 ms, SD = 451.5). The main effect of Diagnosis and the Diagnosis by Genotype interaction were not significant.
Complementary analyses used anxiety scores instead of Diagnostic groups. After age correction, the correlation of RT with SCAREDpc significantly differed between Genotype groups (F = 7.26, df = 1,62, p = 0.009). However, when computed for each group separately, neither of the correlations of RT with anxiety was significant. The low-expresser group showed a non-significant positive correlation, while the high-expresser showed a non-significant negative correlation. Findings were similar with age uncorrected (main effect of Genotype: F = 6.89, df = 1,63; p = 0.01).
3.4. Reaction time (RT) and lambda
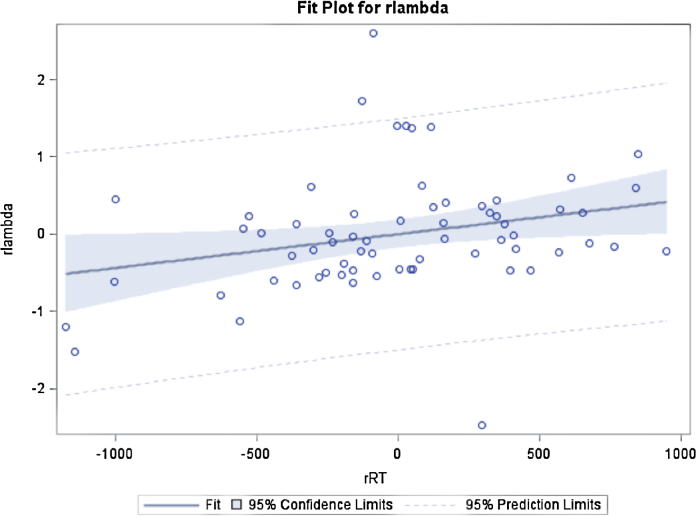
RT for valid trials across bet and pass trials and corrected for age, was positively correlated with lambda (F = 4.8, df = 1,63, p = 0.032) (see Fig. 4). The more loss-averse the participants were, the slower they were. This relationship was not modulated by the type of selection (bet vs. pass; decision*lambda, F = 1.86, df = 1,63, p = 0.18) or by anxiety level (SCAREDpc*lambda F = .20, df = 1,62, p = .65). For completeness, when not corrected for age, the correlation of RT with lambda became not significant (F = 1.53, df = 1,63, p = 0.13).
Fig. 4.
Correlation between reaction time residuals (controlled for age, rRT) and lambda residuals (controlled for age, rlambda) across the whole sample (r = 0.27, p = 0.03, n = 66).
Finally, we repeated all previous analyses after adding RT as a nuisance covariate. The two-way ANCOVA with age and RT as covariates was still significant for the Genotype*Diagnosis interaction on lambda (F = 4.04, df = 1,60, p = 0.049), the main effect of Genotype was a trend (F = 3.65, df = 1,60, p = 0.06), and Diagnosis was not significant (F = 0.65, df = 1,60, p = 0.42). Findings were similar when not correcting for age. After decomposing the Genotype*Diagnosis interaction by conducting an ANCOVA in each diagnostic group, findings were similar to when uncorrected for RT, i.e., showing a significant effect of Genotype on lambda in the anxious group and no effect in the healthy group. Removing the age covariate did not modify these findings.
4. Discussion
Our purpose was 4-fold, to (1) investigate loss aversion in adolescents, (2) compare levels of loss aversion between clinically anxious and healthy adolescents, (3) examine the modulation of loss aversion by the 5HTTPLR variation, (4) particularly as a function of Diagnosis status.
First, we demonstrate that adolescents, like adults, are more sensitive to losses than gains. We also show that levels of loss aversion correlate with age, such that older participants are more loss-averse than their younger counterparts. This latter age-related finding may account to some extent for the relatively lower lambda mean score of 1.4 in this group of adolescents when compared to the typical lambda of 2 described in adults who may continue to grow more loss averse with age (Novemsky and Kahneman, 2005). The potentially lower loss aversion index in adolescents relative to adults may also reflect the higher level of risk-taking behavior in adolescence. This finding is discrepant with the one study comparing lambda in 18 adolescents (15.5 years old) and 16 adults (28 years old) who performed a variant of the mixed-gambles task in the scanner (Barkley-Levenson et al., 2013). This study found a lower lambda than expected in adults, with no age-group differences (lambda adolescent mean = .99, adult mean = 1.1). This negative finding may reflect the relatively small sample sizes. Alternatively, the finding could relate to the context in which the task was performed, i.e., reclining in a scanner. Although a longitudinal study would be the most valid way to examine developmental changes in loss aversion, the present work in 66 adolescents establishes the presence of a proclivity for loss aversion that increases with age. For completeness, we also examined how the effect of age on loss aversion was modulated by genotype and diagnostic status. These exploratory analyses revealed that the correlations of age with lambda differed significantly by genotype, revealing a strong positive correlation in high-expressers (LaLa) and no significant association in low-expressers (S/Lg). Of note, mean age and age range were similar in both genotype groups. In contrast, diagnostic status did not modulate the relation of age with lambda. Because of the relatively small number of subjects, it is premature to interpret these data, particularly in the absence of similar data in the literature. However, the potential role of SERT genotype on the development of loss aversion between ages 8 and 17 years could be examined in relationship to changes in impulsivity and risk-taking over this age range. The low-expression genotype (S), already shown to potentially predispose to higher impulsivity (e.g., Beitchman et al., 2003, Curran et al., 2005, Manor et al., 2001, Retz et al., 2008, Retz et al., 2002, Seeger et al., 2001, Zoroglu et al., 2002), might also contribute to risk-taking by being associated with a lack of increase in loss aversion with age.
The second main finding, against prediction, suggests that levels of loss aversion do not differ between highly anxious and healthy adolescents. Furthermore, lambda was not correlated with anxiety severity (SCAREDpc). This finding is at odds with the expectation that loss aversion might be a facet of harm avoidance, a well-recognized feature of anxiety (Lorian and Grisham, 2010, Maner et al., 2007, Mueller et al., 2010). The next set of findings may shed light on this surprising result by suggesting that the absence of diagnostic-group differences might be related to the variability of loss aversion across anxious individuals, which identifies a subgroup of risk-prone anxious adolescents, as described in previous work (Erwin et al., 2003, Kachin et al., 2001, Kashdan et al., 2006, Kashdan et al., 2008, Kashdan and Hofmann, 2008).
Our third and fourth questions are clarified by the significant interaction of Diagnosis (anxious vs. healthy) by Genotype (high- vs. low-expresser) on lambda and RT. These two interactions indicate that the SERT genotype modulates lambda and RT in anxious patients, but not in healthy adolescents. The anxious adolescents who are also high-expressers (LaLa) execute their decision significantly faster than the other three groups, suggesting that these adolescents also manifest impulsivity. Taken together, these findings identify a subgroup of anxious adolescents who, by virtue of their genotype (5HTTLPR LaLa high-expression), appears to be distinctly low in risk aversion, and fast in making a decision between risky and safe options. The low-expression (S/Lg carriers) anxious group did not differ from the other two healthy subgroups, who themselves did not differ from one another. The group of anxious adolescents, homozygous carriers of the high-expression 5HTTPLR alleles, and who manifest loss-insensitivity (low lambda) and impulsivity (fast reaction time) may represent a subgroup of anxious individuals who are vulnerable to comorbid disorders associated with impulsivity and risky decision, such as substance abuse (Compton et al., 2007, Pasche, 2012). Such comorbidity significantly worsens prognosis and adds substantial complexity to the treatment of anxiety disorders. Conceivably, this risk-taking anxious subgroup may not respond optimally to pharmacotherapy with Selective Serotonin Reuptake Inhibitors. This notion could be critical for treatment planning. However, much work is still needed to validate this subgroup before applying this finding to clinical practice.
A number of limitations need to be considered. First, when decomposed by Diagnosis (anxious, comparison) and Genotype (high-expressers, low-expressers), the sample sizes become relatively small, which may have limited our power to detect significant effects, such as higher loss aversion in low-expresser anxious individuals relative to the healthy group. However, despite this limitation, a significant genotype by diagnosis interaction effect was observed, which was particularly relevant to the question regarding risk factors for comorbid externalizing disorders in anxious adolescents. Nevertheless, this finding should be viewed with caution, since the likelihood of spurious findings also increases with small sample sizes. Second, the anxious group included adolescents diagnosed with a variety of anxiety disorders, which, while matching the most typical presentation of clinical anxiety (Kessler and Wang, 2008, Merikangas and Kalaydjian, 2007, Swinbourne et al., 2012) may have introduced noise to the data. However, such diagnostic heterogeneity might have resulted in providing data on a core behavioral substrate of anxiety present across the various disorders. For completeness, we nevertheless conducted an exploratory ANOVA comparing GAD (n = 16), SocPh (n = 7) and SAD (n = 11) patients, and found no Diagnosis effects on lambda or reaction time (results not shown). Third, age differed significantly across groups, and was correlated with lambda, our main variable of interest. Although we controlled for age in all analyses, the group difference in age remains a limitation, as we may not have been able to control for potential non-linear effects of age. Finally, the implication of the present findings for substance abuse vulnerability cannot be directly assessed in this study of young adolescents, because substance use was an exclusion criterion.
Despite these limitations, as a preliminary study, the present findings can inform future work that begins to probe the neurobiology of a subset of anxiety disorders potentially at risk for severe comorbid disorders related to risk-taking and impulsivity.
Conflict of interest
None of the authors have any actual or potential conflict of interest.
Acknowledgements
We would like to thank Nevia Pavletic for her help in preparing the manuscript.
References
- Barkley-Levenson E.E., Van Leijenhorst L., Galvan A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Developmental Cognitive Neuroscience. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman J.H., Davidge K.M., Kennedy J.L., Atkinson L., Lee V., Shapiro S. The serotonin transporter gene in aggressive children with and without ADHD and nonaggressive matched controls. Annals of the New York Academy of Sciences. 2003;1008:248–251. doi: 10.1196/annals.1301.025. [DOI] [PubMed] [Google Scholar]
- Bengel D., Greenberg B.D., Cora-Locatelli G., Altemus M., Heils A., Li Q. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Molecular Psychiatry. 1999;4(5):463–466. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal s., Brent D., Cully M., Balach L., Kaufman J. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–5553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Compton W.M., Thomas Y.F., Stinson F.S., Grant B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Curran S., Purcell S., Craig I., Asherson P., Sham P. The serotonin transporter gene as a QTL for ADHD. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005:42–47. doi: 10.1002/ajmg.b.30118. 134B(1) [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropschologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B.A., Heimberg R.G., Schneier F.R., Liebowitz M.R. Anger experience and expression in social anxiety disorder: pretreatment profile and predictors of attrition and response to cognitive-behavioral treatment. Behavior Therapy. 2003;34(3):331–350. [Google Scholar]
- Gonda X., Fountoulakis K.N., Juhasz G., Rihmer Z., Lazary J., Laszik A. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. European Archives of Psychiatry and Clinical Neuroscience. 2009;259(2):106–113. doi: 10.1007/s00406-008-0842-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University, Department of Sociology; New Haven, CT: 1975. Four-factor Index of Social Status. (unpublished manuscript) [Google Scholar]
- Hu X.Z., Lipsky R.H., Zhu G., Akhtar L.A., Taubman J., Greenberg B.D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachin K.E., Newman M.G., Pincus A.L. An interpersonal problem approach to the division of social phobia subtypes. Behavior Therapy. 2001;32(3):479–501. [Google Scholar]
- Kahneman D., Lovallo D. Timid choices and bold forecasts – a cognitive perspective on risk-taking. Management Science. 1993;39(1):17–31. [Google Scholar]
- Kahneman D., Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- Kashdan T.B., Collins R.L., Elhai J.D. Social anxiety and positive outcome expectancies on risk-taking behaviors. Cognitive Therapy and Research. 2006;30(6):749–761. [Google Scholar]
- Kashdan T.B., Elhai J.D., Breen W.E. Social anxiety and disinhibition: an analysis of curiosity and social rank appraisals, approach-avoidance conflicts, and disruptive risk-taking behavior. Journal of Anxiety Disorders. 2008;22(6):925–939. doi: 10.1016/j.janxdis.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan T.B., Hofmann S.G. The high-novelty-seeking, impulsive subtype of generalized social anxiety disorder. Depression and Anxiety. 2008;25(6):535–541. doi: 10.1002/da.20382. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Wang P.S. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual Review of Public H. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Lesch K.P., Bengel D., Heils A., Sabol S.Z., Greenberg B.D., Petri S. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lorian C.N., Grisham J.R. The safety bias: risk-avoidance and social anxiety pathology. Behaviour Change. 2010;27(1):29–41. [Google Scholar]
- Maner J.K., Gailliot M.T., Butz D.A., Peruche B.M. Power, risk, and the status quo: does power promote riskier or more conservative decision making? Personality & Social Psychology Bulletin. 2007;33(4):451–462. doi: 10.1177/0146167206297405. [DOI] [PubMed] [Google Scholar]
- Manor I., Eisenberg J., Tyano S., Sever Y., Cohen H., Ebstein R.P. Family-based association study of the serotonin transporter promoter region polymorphism (5-HTTLPR) in attention deficit hyperactivity disorder. American Journal of Medical Genetics. 2001;105(1):91–95. [PubMed] [Google Scholar]
- Merikangas K.R., Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Current Opinion in Psychiatry. 2007;20(4):353–358. doi: 10.1097/YCO.0b013e3281c61dc5. [DOI] [PubMed] [Google Scholar]
- Mueller E.M., Nguyen J., Ray W.J., Borkovec T.D. Future-oriented decision-making in Generalized Anxiety Disorder is evident across different versions of the Iowa Gambling Task. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(2):165–171. doi: 10.1016/j.jbtep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Muris P., Merckelbach H., Schmidt T., Mayer B. The revised version of the Screen for Child Anxiety Related Emotional Disorders (SCARED-R): factor structure in normal children. Personality and Individual Differences. 1999;26(1):99–112. [Google Scholar]
- Novemsky B.M., Kahneman D. The boundaries of loss aversion. Journal of Marketing Res. 2005;42:119–128. [Google Scholar]
- Pasche S. Exploring the comorbidity of anxiety and substance use disorders. Current Psychiatry Reports. 2012;14(3):176–181. doi: 10.1007/s11920-012-0264-0. DOI 10.1007/s11920-012-0264-0. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Ernst M., Leibenluft E. Imaging-genetics applications in child psychiatry. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(8):772–782. doi: 10.1016/j.jaac.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retz W., Freitag C.M., Retz-Junginger P., Wenzler D., Schneider M., Kissling C. A functional serotonin transporter promoter gene polymorphism increases ADHD symptoms in delinquents: interaction with adverse childhood environment. Psychiatry Research. 2008;158(2):123–131. doi: 10.1016/j.psychres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Retz W., Thome J., Blocher D., Baader M., Rosler M. Association of attention deficit hyperactivity disorder-related psychopathology and personality traits with the serotonin transporter promoter region polymorphism. Neuroscience Letters. 2002;319(3):133–136. doi: 10.1016/s0304-3940(01)02575-7. [DOI] [PubMed] [Google Scholar]
- Seeger G., Schloss P., Schmidt M.H. Functional polymorphism within the promotor of the serotonin transporter gene is associated with severe hyperkinetic disorders. Molecular Psychiatry. 2001;6(2):235–238. doi: 10.1038/sj.mp.4000820. [DOI] [PubMed] [Google Scholar]
- Sen S., Burmeister M., Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004:85–89. doi: 10.1002/ajmg.b.20158. 127B(1) [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neuroscience Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swinbourne J., Hunt C., Abbott M., Russell J., St Clare T., Touyz S. The comorbidity between eating disorders and anxiety disorders: prevalence in an eating disorder sample and anxiety disorder sample. Australian and New Zealand Journal of Psychiatry. 2012;46(2):118–131. doi: 10.1177/0004867411432071. [DOI] [PubMed] [Google Scholar]
- Tom S.M., Fox C.R., Trepel C., Poldrack R.A. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. Journal of Risk and Uncertainty. 1992;5:297–323. [Google Scholar]
- Weschler D. The Psychological Corporation; San Antonio: 1999. Weschler abbreviated scale of intelligence (WASI) [Google Scholar]
- Xu K., Ernst M., Goldman D. Imaging genomics applied to anxiety, stress response, and resiliency. Neuroinformatics. 2006;4(1):51–64. doi: 10.1385/NI:4:1:51. [DOI] [PubMed] [Google Scholar]
- Zoroglu S.S., Erdal M.E., Alasehirli B., Erdal N., Sivasli E., Tutkun H. Significance of serotonin transporter gene 5-HTTLPR and variable number of tandem repeat polymorphism in attention deficit hyperactivity disorder. Neuropsychobiology. 2002;45(4):176–181. doi: 10.1159/000063667. [DOI] [PubMed] [Google Scholar]