Highlights
-
•
We use two methods: event-related potentials and eye tracking, to examine face processing in 7-month old infants.
-
•
Our specific focus concerns the impact of extensive visual experience on perceiving face gender and recognizing faces.
-
•
We found that female faces elicited a larger N290 (a face sensitive ERP component) than did male faces.
-
•
The N290 was more negative for novel female faces than primed female faces but no differences were found between male faces.
-
•
Behaviorally infants showed evidence of discriminating both male and female faces.
Keywords: Face processing, Infants, Experience, Event-related potentials, Eye-tracking
Abstract
The goal of the present study was to investigate infants’ processing of female and male faces. We used an event-related potential (ERP) priming task, as well as a visual-paired comparison (VPC) eye tracking task to explore how 7-month-old “female expert” infants differed in their responses to faces of different genders. Female faces elicited larger N290 amplitudes than male faces. Furthermore, infants showed a priming effect for female faces only, whereby the N290 was significantly more negative for novel females compared to primed female faces. The VPC experiment was designed to test whether infants could reliably discriminate between two female and two male faces. Analyses showed that infants were able to differentiate faces of both genders.
The results of the present study suggest that 7-month olds with a large amount of female face experience show a processing advantage for forming a neural representation of female faces, compared to male faces. However, the enhanced neural sensitivity to the repetition of female faces is not due to the infants’ inability to discriminate male faces. Instead, the combination of results from the two tasks suggests that the differential processing for female faces may be a signature of expert-level processing.
1. Introduction
Faces are ubiquitous stimuli in our social world, and are particularly salient for infants. Indeed, even newborn infants show a preference for faces and face-like stimuli, compared to other visual objects (Johnson and Morton, 1991, Maurer, 1983, Mondloch et al., 1999, Valenza et al., 1996). Infants’ intrinsic preference for face-like stimuli and the pervasiveness of faces in infants’ visual environment contributes to a rapid development of face processing abilities over the first few months of life. For example, work by Barrera and Maurer (1981) showed that starting at around 3 months of age infants are able to distinguish their mother's face from that of another woman in photographs, and are also able to reliably discriminate female faces that are unfamiliar to them.
Around this same age, infants also begin to show the ability to create distinct face categories along different dimensions. Quinn et al. (2002) tested 3 to 4 month old infants using behavioral looking paradigms to examine whether young infants could categorize faces according to the gender of the face. These authors were also interested in whether the experiences accrued with faces influenced infants’ behavior, and for this they tested infants who were reared primarily with female or male caregivers. They found that female-reared infants showed a spontaneous preference for female faces, while male-reared infants showed a spontaneous preference for male faces. Analogous to their gender preference, infants as young as 3 months show a preference for faces that belong to the racial/ethnic group that they see most often (Bar-Haim et al., 2006, Kelly et al., 2005). Taken together, these findings show that by 3 months of age infants are already relying on experience with the visual environment to shape their behavior and to form basic categories along different dimensions of face-specific features.
Extensive experience with a specific category of faces also helps infants develop better discrimination abilities for individuals within the same category (Quinn et al., 2002, Scott and Monesson, 2009). Quinn et al. (2002) familiarized 3 to 4 month old “female expert” infants with either a female face or a male face. Successively they tested them with a pair of faces, either females or males, which contained the previously familiarized face and a novel face. They found that these infants showed a significant novelty preference when tested with female faces, but not with male faces, suggesting that they were able to discriminate between female faces, but not between male faces.
The influence of experience on face processing is present throughout the life-span, and gives rise to both behavioral and neural effects. There is an extensive literature documenting differences in the accuracy of identity recognition when adult observers are presented with faces belonging to a racial/ethnic group with which they have little visual experience. For example, observers are better at discriminating between members of the category with which they are more familiar (e.g., Sporer, 2001). Recent studies using event-related potentials (ERP) have also shown that differential experience with face categories produces category-sensitive neural signatures in adults (Balas and Nelson, 2010, Caldara et al., 2003, Stahl et al., 2008, Tanaka and Pierce, 2009).
Despite a relatively extensive literature on the behavioral manifestations of experience in infants (see Pascalis et al., 2005 for a review), less is known about its influence on the neural correlates of face processing. Based on the findings from the adult literature, and the similarities in behavioral effects observed across development, one might predict that in infants, extensive experience with a specific category of faces would produce specific neural signatures in response to the category of expertise. The goal of the present study is to further investigate experience-dependent neural and behavioral responses in the context of face gender in infants using ERPs and a preferential looking paradigm.
With regard to the processing of faces, there are several relevant components that have been identified in infants: the N290, the P400, the Negative Component (NC), and the Positive Slow Wave (PSW). The N290 is a negative component most prominent over posterior electrodes. This component shows an enhanced response to faces starting in infants as young as 3 months (Halit et al., 2004), and by 12 months of age shows a differential response to upright and inverted faces (Halit et al., 2004). The P400 is a positive component strongest over posterior electrodes that shows faster latencies to faces than objects in infants as young as 6 months of age (de Haan & Nelson, 1999). Moreover, this component shows amplitude modulations in response to the picture-plane inversion of faces in infants as young as 3 months of age (de Haan et al., 2002, Halit et al., 2004). The NC is a negative deflection strongest over fronto-central electrodes. With regard to face processing, the NC has been related to the allocation of attention, and it has been shown to differentially respond to an infant's own mother's face, compared to a stranger's face in infants as young as 6 months of age (de Haan & Nelson, 1997). Finally, the PSW is observed over posterior electrodes, and has been associated with the process of creating and updating perceptual representations of faces (de Haan & Nelson, 1999), and it is found in infants as young as 3 months of age.
One might expect that all of the ERP components discussed above may show some degree of experience-dependent modulations based on the behavioral effects observed in infants, but little is known about these changes. One of the few studies that investigated the impact of experience on infants’ neural responses was carried out by Moulson et al. (2011). These authors examined the role of experience with a 3-D model of a female face in 3 month olds. They tested infants who either received 1 month of in-home familiarization with a model, or a 1.5-minute in-lab familiarization with a model. During the experiment, the infants were shown pictures of either the model they were familiar with, or a different 3-D model while ERPs were recorded. Moulson et al. (2011) found that infants who were familiarized with a model for a 1 month period showed larger P400 amplitude and a more negative NC to the familiar stimulus compared to the novel stimulus, while the infants who were introduced to a model only during their in-lab visit showed the opposite pattern. These results are consistent with the notion that neural responses are sensitive to experience from an early age.
In another recent study, experience-dependent effects have also been shown at the category-level in infants. Balas et al. (2011) used ERP to test infants’ neural responses to faces of either their own race or a different race. These authors tested 9 month-old Caucasian infants using Caucasian and African faces, and found that these infants, who had experience primarily with Caucasian faces, produced a significantly larger N290 in response to Caucasian faces, compared to African faces.
The results of the studies discussed above provide evidence for experience-dependent modulation of neural responses from an early age. However, it has yet to be established whether infants with extensive experience with a specific category of faces show better discrimination abilities for exemplars of within the category of expertise, akin to what is observed in adults. In order to answer this question we used male and female faces, because most infants raised by female caregivers tend to have significantly more experience with female faces, compared to male faces (Rennels & Davis, 2008).
To examine experience-dependent signatures of face gender processing we tested 7-month-old infants using both an ERP paradigm and a preferential looking paradigm. The former included a priming design in which infants saw alternating female and male faces, in order to study the neural responses related to their recognition of individual faces. The behavioral experiment employed a visual-paired comparison (VPC) paradigm, which relies on the analysis of looking times to measure discrimination abilities. In this paradigm infants studied a single face, either male or female, and were subsequently presented with the familiarized face aside a new face of the same gender. The inclusion of both paradigms in the same experimental design was motivated by the desire, to better understand the nature of experience-dependent mechanisms in infancy both neurally and behaviorally.
Given prior literature, we would expect infants who are primarily exposed to faces of a single gender to show larger amplitude in ERP components to such faces. Moreover, infants may also show sensitivity to the repetition of facial identities of the more familiar gender. Behaviorally, given the nature of the paradigm chosen, we may expect to find different looking times between the novel and familiar face of the gender with which infants have more experience. A significant discrepancy in looking time would be indicative of the infants’ discrimination abilities. However, it would not be surprising to find equal discrimination abilities across face genders, because in our behavioral paradigm infants were presented with a face for a relatively long period of time, resulting in an easier task, compared to when electrophysiological measures were recorded.
2. Methods
2.1. Participants
Eighty 7 month-old infants were recruited to participate in the study. All infants were born full-term and had no known peri- or post-natal complications (M age = 218 days, SD = 9.0, range 203–246). Informed consent according to the guidelines of Boston Children's Hospital IRB was obtained from the parents. Additionally the parents filled out a questionnaire designed to gather information about their baby's experience with male and female faces, by measuring the daily amount of waking hours spent, on average, with either male or female caregivers, or both. Given the objective of the present study, 58 infants were selected to be part of the final study sample based on their extensive amount of experience with a female primary caregiver. The selection criteria was that infants had to spend >70% of their waking hours with a female (mother or other caregiver). This criterion was based on recent work showing that infants raised by primary female caregivers see, on average, female faces 70% of the time (Rennels & Davis, 2008).
From the study sample, sixteen participants (5 females) were included in the final study sample for the ERP experiment. The additional 42 infants tested were excluded due to the following reasons: excessive eye and/or body movements that resulted in recording artifacts (n = 36), or fussiness that resulted in too few trials being recorded (n = 6). The low number of infants accepted included in the analyses is likely due to the 2 × 2 design adopted in our study, which is relatively uncommon in the infant ERP literature. The full factorial design implemented in the present study resulted in higher than usual requirements for artifact-free trials, which in turn diminished the sample size.
From the study sample, twenty-three participants (9 female) were included in the final study sample for the eye tracking experiment. The additional 35 infants tested were excluded due to the following reasons: did not complete the eye tracking experiment (n = 24), produced insufficient data (n = 9), or evidence of a side bias (n = 2, see eye tracking analysis below).
Due to variable attrition rates across the ERP and eye tracking experiments only nine infants provided usable data for both experiments. In order to maximize the amount of available data in each set of analyses we included all infants who, (a) spent >70% of their waking hours with a female caregiver, and (b) provided useable data for the respective experiment.
2.2. Stimuli
Stimuli were color images of female and male faces drawn from several publicly available stimulus sets, including the NimStim set of images (Tottenham et al., 2009), the Productive Aging Lab Face Database (Minear & Park, 2004), and the FERET database of facial images collected under the FERET program, sponsored by the DOD Counterdrug Technology Development Program Office (Phillips et al., 1998, Phillips et al., 2000). All faces posed a neutral expression and had been rated on perceived age, attractiveness, and gender by a group of adult observers and chosen from a larger set of images such that there were no significant differences on any of the rated dimensions.
For the ERP portion of the experiment we used 40 female and 40 male faces, and each identity was repeated only once.
For the eye tracking portion of the experiment we used 2 female and 2 male faces; face pairs were rated for similarity by a group of adult observers, such that the experimental pairs were chosen to share overall shape, eye color, hair color and expression. The faces selected for the eye tracking study were not included as stimuli in the ERP task.
In both ERP and eye tracking experiments the stimuli were presented on a black background and subtended 9.2° × 13.6° of visual angle.
2.3. Procedure
The experimental session consisted of two experiments: an ERP experiment and an eye tracking experiment. The two tasks were always presented in the same order with the ERP task coming first. This order was motivated by wanting to maximize the number of trials that the infants could tolerate in the ERP task, in order to have sufficient power for statistical analyses of these data.
2.3.1. ERP experiment
Continuous EEG was recorded during a priming paradigm that included male and female faces. The paradigm was divided into 8 blocks that contained 20 stimulus presentations each. Within each block we created 5 sequences of 4 images in the following sequence: face 1, face 2, face 1, face 2. Within each sequences the faces could be all female, all male, or alternating male and female. These sequences were created in order to control for any possible contextual effects that could be affecting the neural responses to the repetition of specific identities. That is, we wanted to ensure that differences in similarities between faces of the same gender compared to faces of a different gender did not have a differential impact on the neurophysiological components elicited by the second presentation of a specific identity. The order of these sequences was randomized across the experiment. See Fig. 1 for an example of a sequence.
Fig. 1.
Example of a stimulus block in the ERP paradigm with alternating female and male faces.
Image presentation was experimenter-controlled, such that a stimulus would appear on the screen only if the infant, who was monitored using a video camera, was looking at the computer monitor. Each face image was presented for 500 ms, followed by a blank screen presented for a varying amount of time, depending on the infant's attention. The minimum inter-stimulus interval was 2000 ms. Image presentation was achieved using E-Prime (Psychological Software Products, Harrisburg, PA).
2.3.2. ERP recording and analysis
Continuous scalp electroencephalogram (EEG) was recorded from a 128-channel HydroCel Geodesic Sensor Net1 (Electrical Geodesic Inc., Eugene, OR) that was connected to a NetAmps 200 amplifier (Electrical Geodesic Inc., Eugene, OR) and referenced online to a single vertex electrode (Cz). Channel impedances were kept at or below 50 kΩ. Analog voltages were bandpass filtered at 0.1–100 Hz, digitized at 250 Hz and stored on a disk. Data were preprocessed offline using NetStation 4.4.1 (Electrical Geodesic Inc., Eugene, OR).
The EEG signal was segmented to 1500 ms post-stimulus onset, with a baseline period beginning 100 ms prior to stimulus onset. Data segments were filtered using a 30-Hz low-pass elliptical filter and baseline-corrected using mean voltage during the 100 ms pre-stimulus period. Automated artifact detection was applied to the segmented data in order to detect individual sensors that showed >200 μV voltage changes within the segment period. The entire trial was excluded if more than 18 sensors had been rejected. Data segments were then inspected manually to confirm the results of the automatic artifact detection algorithms. Segments containing eye blinks, eye movements, or high frequency noise were also rejected. Bad segments identified by either procedure were excluded from further analysis. Of the remaining trials, individual channels containing artifact were replaced using spherical spline interpolation. Average waveforms were generated separately for the novel and repeated male and female faces within each participant, and data were re-referenced to the average reference. The mean number of trials per condition average was 16 (SD = 6.4, range 8–33).
Inspection of the grand-averaged waveforms revealed two well-defined components that were prominent over occipito-temporal scalp: N290 and P400. Based on grand-averaged data and individual data, the N290 was analyzed within a time window of 140–250 ms and the P400 was analyzed within a time window of 300–600 ms. Two components that were maximal over fronto-central scalp locations were also observed: NC (time window of 300–600 ms) and positive slow wave (PSW, time window of 1000–1500 ms). Electrode groupings were selected based on visual inspection of both the grand-averaged and individual waveforms. Nine occipital electrodes were identified for the N290 and P400 (69, 70, 74, 71, 75, 76, 82, 83, 89), and twelve fronto-central electrodes were identified for the NC and PSW (30, 36, 37, 7, 31, 55, vREF, 80, 106, 87, 104, 105). Electrode locations were subsequently separated into left, midline, and right regions for analysis. For the N290 and P400 the left region contained electrodes 69, 70 (O1),2 and 74; the midline region contained electrodes 71, 75 (Oz) and 76; the right region contained electrodes 82, 83 (O2), and 89. For the NC and PSW the left region contained electrodes 30 (C1), 36 (C3), 37 (Cp1); the midline region contained electrodes 7, 31, 55 (Cpz), vREF (Cz), 80, and 106; the right region contained electrodes 87 (Cp2), 104 (C4), and 105 (C2). Mean amplitude was extracted for all four components. Both time windows and the electrode groupings chosen are comparable to those selected in recent ERP investigations of face processing in infants (e.g., Balas et al., 2011, Halit et al., 2004).
2.3.3. Eye tracking experiment
This experiment consisted of a visual-paired comparison paradigm (VPC). The VPC task consisted of two problem sets: one problem set included female faces, while the other problem set included male faces. The order of presentation of male and female faces was counterbalanced across subjects. See Fig. 2 for examples of VPC stimulus pairings.
Fig. 2.
Example of VPC problem sets for panel (A) female faces, and panel (B) male faces.
The experiment began with a 5-point calibration procedure that was used to ensure accurate tracking of the infant's eyes. Each problem set started with the presentation of a single face that remained on the screen until the infant had looked at it for a total of 20 s. The familiarization period was followed by a brief attention-getting video (∼1 s), in order to make sure that the infant's attention remained on the screen. Successively the familiarization face was presented side-by-side with a novel face for 5 s. After the images disappeared from the screen a new attention-eliciting stimulus was shown for 1 s. Lastly the familiarization face and the novel face were presented again, but in reversed position compared to the first presentation, and remained on the screen for 5 s.
The calibration procedure and stimuli were presented using Tobii Studio (Tobii Technology AB, Sweden); for the entire duration of this experiment eye movement data were recorded at 60-Hz using a Tobii T120 (Tobii Technology AB, Sweden) corneal reflection eye tracker.
2.3.4. Eye tracking data analysis
Eye tracking data was used to measure looking time to each of the stimulus presentations. For each face stimulus, an experimenter manually traced areas of interest (AOI) that covered the entire face oval using Tobii Studio (Tobii Technology AB, Sweden). These AOIs were akin to transparent masks placed over the face stimuli that allowed Tobii Studio to distinguish looks directed at each face stimulus from looks directed at other portions of the screen. Within these AOIs we measured the total amount of looking time produced by each infant during the familiarization phase and the two test phases. In order to assess for potential side bias, we computed an index by calculating the percentage of time that each infant looked at the AOIs on either side of the screen during the test phases. If infants showed more than a 90% side bias, they were excluded from further analyses (n = 2).
3. Results
3.1. ERP data
Mean amplitude for each component was analyzed using repeated-measures analyses of variance (ANOVA). Within-subjects factors included repetition (novel, primed), face gender (male, female). Electrode location (left, right, midline), which was originally included as a within-subject factor in each model, was dropped from the models reported here because there were no significant effects or significant interactions that included this factor. Furthermore, participant gender was included as a between-subjects factor in each model, and subsequently dropped when it was not significant. Greenhouse–Geisser corrections were applied when the assumption of sphericity was violated. When significant (p ≤ .05) main or interaction effects emerged, post hoc comparisons were conducted and a Bonferroni correction for multiple comparisons was applied.
3.1.1. N290
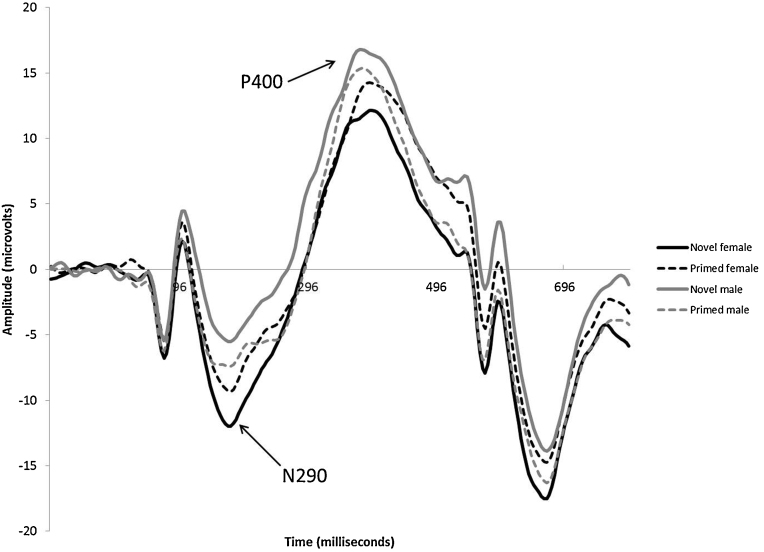
Analysis of N290 mean amplitude revealed a significant main effect of face gender, F(1,15) = 7.069, p = .018, whereby female faces (M = −8.15 μV, SD = 8.4) elicited significantly larger amplitudes than male faces (M = −5.07 μV, SD = 7.9). There was also a significant face gender × repetition interaction, F(1,15) = 5.054, p = .033 (see Fig. 3) Post hoc comparisons revealed that infants showed a priming effect for female faces only, with a more negative amplitude in response to novel female faces, compared to repeated female faces (novel female, M = −9.40 μV, SD = 8.5; primed female, M = −6.90 μV, SD = 8.8), F(1,15) = 5.264, p = .037. There were no significant differences in the N290 response to novel male (M = −3.80 μV, SD = 6.8) versus primed male faces (M = −6.34 μV, SD = 10.2), F(1,15) = 2.128, p = .165.
Fig. 3.
Grand averaged ERP waveforms showing the N290 and P400 at posterior electrodes (collapsed across region of interest). The x-axis represents latency in milliseconds (ms) and the y-axis represents amplitude in microvolts (μV).
3.1.2. P400
Visual inspection of waveforms revealed that amplitude differences of the P400 may be driven by differences in the preceding N290 component. In order to control for the differences at the N290 in the analysis of the P400, a peak-to-trough subtraction was computed. The difference score variable for the P400 was entered in the analysis of variance. Analyses revealed that after correcting for the preceding N290 effects, there were no main or interaction effects at the P400.
3.1.3. NC
There were no main effects or interaction effects for mean amplitude of the NC.
3.1.4. PSW
There were no main effects or interaction effects for PSW mean amplitude.
3.2. Eye tracking data
Looking times on the visual-paired comparison test trials were analyzed using repeated-measures analyses of variance (ANOVA). Within-subjects factors included test trial (first presentation, second presentation), memory condition (novel, familiar), and face gender (female, male). To clarify, female and male faces were never paired directly in our experimental design.
Participant gender was included as a between-subjects factor in each model, and subsequently excluded because it had no significant impact on the analyses. When significant (p's ≤ .05) main or interaction effects emerged, post hoc comparisons were conducted and corrections for multiple comparisons were applied.
Analyses revealed a significant memory × test trial interaction, F(1,22) = 5.951, p = .023. Post hoc comparisons indicated that infants showed a robust novelty preference on the first presentation of the pair of faces regardless of the gender of the face pair (trial 1 novel face, M = 2015.95 ms, SD = 491.8; trial 1 familiar face, M = 1701.70 ms, SD = 472.0). Follow-ups also showed that looking at the novel face significantly declined from the first presentation (M = 2015.95 ms, SD = 491.8) to the second presentation (M = 1672.02 ms, SD = 643.3), whereas looking at the familiar face stayed consistent from first presentation (M = 1701.70 ms, SD = 472.0) to the second presentation (M = 1846.87 ms, SD = 555.4).
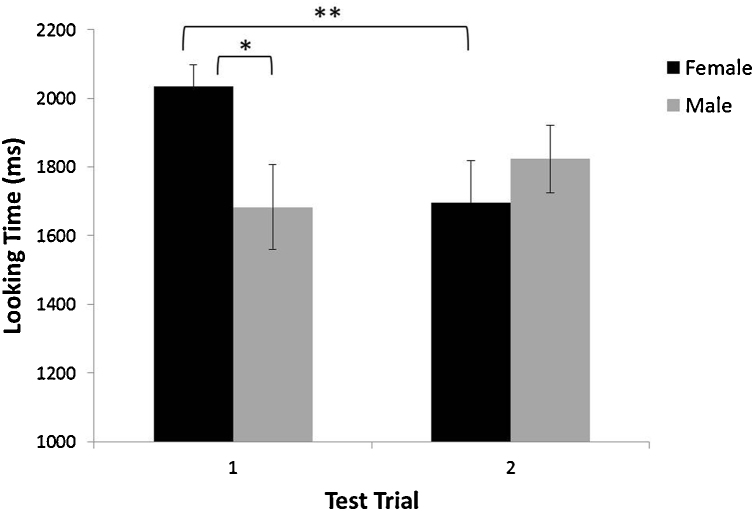
Analyses also revealed a significant face gender × test trial interaction, F(1,22) = 7.220, p = .013 (see Fig. 4). Post hoc comparisons indicated that infants looked longer at female faces (M = 2034.41 ms, SD = 303.1) compared to male faces (M = 1683.23 ms, SD = 590.7) during the first presentation, regardless of whether the face was novel or familiar. Follow-ups also showed that looking at female faces significantly declined from the first presentation (M = 2034.41 ms, SD = 303.1) to the second (M = 1695.91 ms, SD = 576.2), whereas looking at male faces stayed consistent from the first presentation (M = 1683.23 ms, SD = 590.7) to the second (M = 1822.99 ms, SD = 468.5). There were no significant effects of face gender or memory on the second test trial.
Fig. 4.
Face gender × test trial interaction for VPC looking times. Asterisks represent statistical significance. Error bars represent standard errors of the mean.
4. Discussion
The goal of the present study was to investigate the nature of experience-dependent neural and behavioral responses to female and male faces. In order to do this, we tested a group of 7 month-old who had a disproportionate amount of visual experience with female faces compared to male faces. These infants were tested using a priming paradigm while recording EEG, and a visual-paired comparison paradigm.
The results of the ERP paradigm showed that the amplitude of the N290 was larger for female faces compared to male faces. This pattern is similar to what is observed in studies of perceptual expertise in adults. Several authors have shown that the neural responses of adult experts in early ERP components like the N170 and N250 are enhanced in response to objects within their category of expertise, compared to other objects (e.g., Scott et al., 2006a, Tanaka, 2001, Wong et al., 2005). For example, Tanaka and Curran (2001) found that adult dog and bird experts showed an enhanced N170 in response to pictures of dogs, and birds, respectively. These results were interpreted as demonstrative of the emergence of experience-dependent neural tuning. Thus, the increased N290 response observed in the present study in response to female faces, compared to male faces, may be interpreted as a manifestation of increased neural tuning produced by extensive and selective exposure to female faces. In adults enhanced neural tuning has also been linked to the efficient classification of objects within the domain of expertise, which, in turn, facilitates the recognition of individual exemplars (Ullman, 1996 cf. Tanaka & Curran, 2001). This might be the case in infants with extensive experience with a specific category of faces, as the N290 is also sensitive to the repetition of individual female faces, but not to the repetition of individual male faces. Thus 7-month olds infants show neural signatures modulated by the characteristics of their visual environment.
In the present study we did not find any significant effects of the P400. There are several studies in which the authors did not find differences in the P400 between different categories of faces (Balas et al., 2011, de Haan et al., 2002). The present study provides further evidence suggesting that this component is not sensitive to categorical differences between stimuli in infants (Balas et al., 2011). With regard to identity processing, a few studies with infants have shown increased P400 amplitude in response to highly familiar faces, compared to novel faces (e.g., Moulson et al., 2011, Scott et al., 2006b). The lack of priming effects observed in the present study is likely due to the fact that the infants were never extensively familiarized with any specific identity.
No significant effects were found for the NC component. In the literature, several authors have observed amplitude modulations in the NC for own mother's face compared to a stranger face in infants as young as 6 months (de Haan & Nelson, 1997), and Moulson and colleagues showed that 3 month olds who received extensive experience with a 3-D head model produced a larger NC to photographs of the familiar model, compared to photographs of a novel model (Moulson et al., 2011). However, the majority of studies examining the NC have used paradigms that have included stimuli that are highly familiar to the infants at the individual level, which is not the case in the present study. Thus, the lack of modulations for identity at the level of the NC may be related to the brief presentation of each identity, which is unlikely to produce robust familiarity effects in infants.
Lastly, no significant effects were observed for the PSW. There are several studies linking this component to the updating of mental representations (de Haan and Nelson, 1999, de Haan et al., 2003), and to the detection of novelty (de Haan and Nelson, 1997, Gunnar and Nelson, 1994). The PSW can also be modulated by the presentation of personally familiar faces and familiar toys (de Haan and Nelson, 1997, de Haan and Nelson, 1999). However, the paradigm employed in the present study is quite different from prior work that has shown differences in the PSW on the bases of memory. While some studies have used personally familiar stimuli (e.g., de Haan and Nelson, 1997, de Haan and Nelson, 1999), other studies have used oddball-like paradigms in which one stimulus is repeated significantly more than any other (Gunnar and Nelson, 1994, Nelson and Collins, 1991, Wiebe et al., 2006). Therefore, the lack of differences in the PSW observed in the present study is likely due to the nature of our paradigm, in which all faces were shown an equal number of times and they were all novel for the infants.
Overall, the results of the ERP paradigm suggest that significant experiences with faces of a specific gender modulates the neural responses of an early face-sensitive component, comparable to what is observed in the brains of adult subjects who are experts with a specific domain of visual objects. This sensitivity to female faces may result from neural tuning that takes place over the first few months of life. The development of gender-specific neural tuning may, in turn, facilitate the rapid processing of female faces, as shown by the presence of a priming effect for these faces. However, it appears that at 7 months of age infants may still be too young to be able to form stable representation of rapidly presented female faces, as suggested by the lack of modulation in components that are related to deeper aspects of identity processing.
The results of the behavioral experiment provide an important complement to the ERP results discussed above, as one might infer that the lack of neural priming effects for male faces could be related to the inability to discriminate among individuals within this category. However, this does not appear to be the case because in the present experiment 7-month old infants were able to successfully discriminate both female and male faces. This result is not surprising for variety of reasons. First of all, all of the infants in our final sample had some degree of exposure to male faces, because they all lived in households with a male. Thus, they are not entirely novices with regard to male faces. Secondly, in the VPC paradigm infants were familiarized with an individual face for several seconds, which, by this age, may be enough to encode faces with which they have less experience (Moulson et al., 2011). Third, in the test trials the infants were provided the opportunity to compare the familiar and novel faces side by side, which may place less of a burden on their developing memory system than occurs in a priming task, where the stimuli are presented individually, distributed over time. Given that it has been shown in the adult literature that behavioral differences between experts and non-experts tend to be largest in experimental tasks that are more demanding such as a 2-back task (Bukach et al., 2010, Gauthier et al., 2000), it is possible that the lack of significant gender effects in the VPC is due to the relatively easier nature of this task, compared to the priming paradigm.
The present study has several limitations. First of all, the 4-condition design employed in the ERP experiment greatly contributed to the high attrition rate among the infants tested, because relatively few infants provided enough artifact-free trials across all conditions. Secondly, the present study would have greatly benefitted from the presence of a significant group of infants who were raised by male primary caregivers, in order to further validate the impact of experience on neural tuning. However, despite extensive recruiting efforts, very few families with males as primary caregivers participated in the study.
5. Conclusions
In conclusion, the findings from the two paradigms employed in the present study provide evidence that infants who have significant exposure to female faces show neural sensitivity to the repetition of female identities, but not to the repetition of male identities. Such sensitivity can be linked to extensive exposure to multiple exemplars of female faces, and it is not driven by the inability to discriminate the identity of male faces. The sensitivity of the N290 to the repetition of female faces might also be indicative of more rapid processing of these faces made possible by the emergence of specialized neural tuning, linked to the greater visual experience with female faces. Thus we have identified subtle experience-dependent differences in face processing that are not captured by a VPC paradigm. The nature of these differences is consistent with the adult literature and hints at a common developmental trajectory of experience-dependent perceptual processes across the lifespan.
Conflicts of interest
None.
Acknowledgements
The research reported in this manuscript was supported by the NIMH (R01MH078829) (to CAN). The authors are indebted to the many children and families who have graciously given of their time.
Footnotes
Eye electrodes were removed from the nets, in order to be more comfortable for the infants, thus resulting in 124 recording channels.
Electrode locations corresponding to 10–10 system sites are added in parenthesis when available.
References
- Balas B., Nelson C.A. The role of face shape and pigmentation in other-race face perception: an electrophysiological study. Neuropsychologia. 2010;48(2):498–506. doi: 10.1016/j.neuropsychologia.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas B., Westerlund A., Hung K., Nelson Iii C.A. Shape, color and the other-race effect in the infant brain. Developmental Science. 2011;14(4):892–900. doi: 10.1111/j.1467-7687.2011.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Ziv T., Lamy D., Hodes R.M. Nature and nurture in own-race face processing. Psychological Science: A Journal of the American Psychological Society/APS. 2006;17(2):159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Barrera M.E., Maurer D. The recognition of mother's photographed face by the three-month-old infant. Child Development. 1981;52:114–118. [Google Scholar]
- Bukach C.M., Phillips W.S., Gauthier I. Limits of generalization between categories and implications for theories of category specificity. Attention, Perception & Psychophysics. 2010;72(7):1865–1874. doi: 10.3758/APP.72.7.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldara R., Thut G., Servoir P., Michel C.M., Bovet P., Renault B. Face versus non-face object perception and the ‘other-race’ effect: a spatio-temporal event-related potential study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2003;114(3):515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Recognition of the mother's face by six-month-old infants: a neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- de Haan M., Nelson C.A. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- de Haan M., Humphreys K., Johnson M.H. Developing a brain specialized for face perception: a converging methods approach. Developmental Psychobiology. 2002;40(3):200–212. [PubMed] [Google Scholar]
- de Haan M., Johnson M.H., Halit H. Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Gauthier I., Skudlarski P., Gore J.C., Anderson A.W. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Nelson C.A. Event-related potentials in year-old infants: relations with emotionality and cortisol. Child Development. 1994;65(1):80–94. [PubMed] [Google Scholar]
- Halit H., Csibra G., Volein A., Johnson M.H. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(7):1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Morton H. Blackwell; Oxford: 1991. Biology and Cognitive Development: The Case of Face Recognition. [Google Scholar]
- Kelly D.J., Quinn P.C., Slater A.M., Lee K., Gibson A., Smith M., Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8(6):F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D. The scanning of compound figures by young infants. Journal of Experimental Child Psychology. 1983;35(3):437–448. doi: 10.1016/0022-0965(83)90019-x. [DOI] [PubMed] [Google Scholar]
- Minear M., Park D.C. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mondloch C.J., Lewis T.L., Budreau D.R., Maurer D., Dannemiller J.L., Stephens B.R., Kleiner-Gathercoal K.A. Face perception during early infancy. Psychological Science. 1999;10(5):419–422. [Google Scholar]
- Moulson M.C., Shannon R.W., Nelson C.A. Neural correlates of visual recognition in 3-month-old infants: the role of experience. Developmental Psychobiology. 2011;53(4):416–424. doi: 10.1002/dev.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Collins P.F. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27(1):50–58. [Google Scholar]
- Pascalis O., Scott L.S., Kelly D.J., Shannon R.W., Nicholson E., Coleman M., Nelson C.A. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.J., Wechsler H., Huang J., Rauss P. The FERET database and evaluation procedure for face recognition algorithms. Image and Vision Computing. 1998;16(5):295–306. [Google Scholar]
- Phillips P.J., Moon H., Rizvi S.A., Rauss P.J. The FERET evaluation methodology for face recognition algorithms. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2000;22:1090–1104. [Google Scholar]
- Quinn P.C., Yahr J., Kuhn A., Slater A.M., Pascalils O. Representation of the gender of human faces by infants: a preference for female. Perception. 2002;31(9):1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Rennels J.L., Davis R.E. Facial experience during the first year. Infant Behavior & Development. 2008;31(4):665–678. doi: 10.1016/j.infbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.S., Monesson A. The origin of biases in face perception. Psychological Science: A Journal of the American Psychological Society/APS. 2009;20(6):676–680. doi: 10.1111/j.1467-9280.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- Scott L.S., Shannon R.W., Nelson C.A. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy: The Official Journal of the International Society on Infant Studies. 2006;10(2):171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.S., Tanaka J.W., Sheinberg D.L., Curran T. A reevaluation of the electrophysiological correlates of expert object processing. Journal of Cognitive Neuroscience. 2006;18(9):1453–1465. doi: 10.1162/jocn.2006.18.9.1453. [DOI] [PubMed] [Google Scholar]
- Sporer S.L. Recognizing faces of other ethnic groups. Psychology, Public Policy & Law. 2001;7:36–97. [Google Scholar]
- Stahl J., Wiese H., Schweinberger S.R. Expertise and own-race bias in face processing: an event-related potential study. Neuroreport. 2008;19(5):583–587. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- Tanaka J. The entry point of face recognition: evidence for face expertise. Journal of Experimental Psychology. 2001;130(3):534–543. doi: 10.1037//0096-3445.130.3.534. [DOI] [PubMed] [Google Scholar]
- Tanaka J.W., Curran T. A neural basis for expert object recognition. Psychological Science: A Journal of the American Psychological Society/APS. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Tanaka J.W., Pierce L.J. The neural plasticity of other-race face recognition. Cognitive, Affective & Behavioral Neuroscience. 2009;9(1):122–131. doi: 10.3758/CABN.9.1.122. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C.A. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman S. MIT Press; Cambridge, MA: 1996. High-Level Vision: Object Recognition and Visual Cognition. [Google Scholar]
- Valenza E., Simion F., Macchi Cassia V., Umilta C. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22(4):892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- Wiebe S.A., Cheatham C.L., Lukowski A.F., Haight J.C., Muehleck A.J., Bauer P.J. Infants’ ERP responses to novel and familiar stimuli change over time: implications for novelty detection and memory. Infancy: The Official Journal of the International Society on Infant Studies. 2006;9(1):21–44. [Google Scholar]
- Wong A.C., Gauthier I., Woroch B., DeBuse C., Curran T. An early electrophysiological response associated with expertise in letter perception Cognitive. Affective & Behavioral Neuroscience. 2005;5(3):306–318. doi: 10.3758/cabn.5.3.306. [DOI] [PubMed] [Google Scholar]