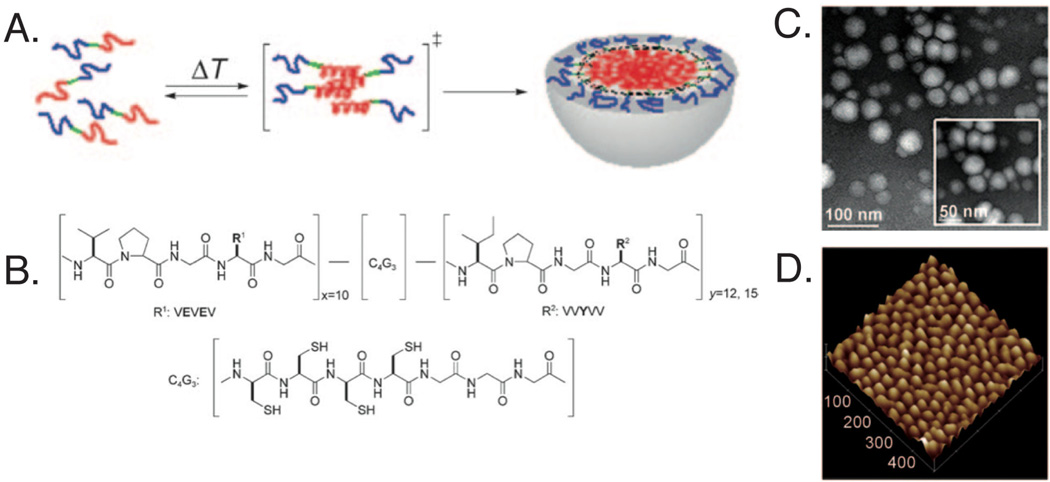
Fig. 3.
Developed elastin-like peptide for self-assembly into micelles. Upon heating, the (A) hydrophobic blocks (red) assemble into micelles with the hydrophilic blocks (blue) creating an outer shell and the cysteine containing regions (green) at the interface. (B) Chemical structure of synthesized amphiphilic diblock polypeptide, where ADP1 (x10y12) and ADP2 (x10y15). Micelles were characterized using (C) transmission electron microscopy and (D) atomic force microscopy, with ADP1 forming ~ 28 nm spheres with low polydispersity. Reprinted with permission from [70]. Copyright 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.