Figure 1.
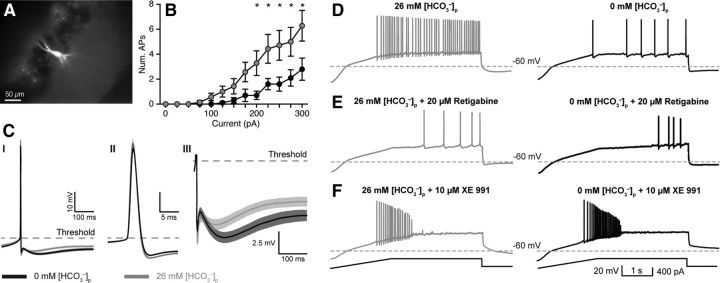
Intracellular HCO3− regulates intrinsic excitability via KCNQ channel activation. A, Whole-cell current-clamp recordings were made from CA3 PCs. A subset of recorded neurons was filled with AlexaFluor 488 (50 μm) to confirm CA3 PC morphology. B, Current steps (300 ms) evoked fewer APs with 0 mm [HCO3−]p, black traces versus 26 mm [HCO3−]p, gray traces (n = 10, p = 0.01, RM two-way ANOVA; asterisks indicate statistically significant post hoc paired tests). C, Spontaneous AP waveforms recorded with 0 or 26 mm [HCO3−]p revealed significantly enhanced mAHP (CI, CIII) in 0 mm [HCO3−]p, whereas AP amplitude, threshold, and half-width were unaltered (CII). D, Current ramp protocols (F, bottom) also revealed reduced AP generation in 0 versus 26 mm [HCO3−]p, indicating reduced excitability. E, The KCNQ channel activator retigabine (20 μm) reduced the number of APs/ramp in 26 mm [HCO3−]p to levels comparable to 0 mm [HCO3−]p. F, In contrast, the KCNQ channel antagonist XE 991 (10 μm) increased the number of APs in both 0 and 26 mm [HCO3−]p and removed the difference between conditions. Data shown as mean ± SEM.