Abstract
Incidences of sand storms have increased in recent years and there is evidence that these dusts can move across long distances. Sand dusts have different adverse effects on health, but one of the most important of them is pulmonary disease. After inhalation of dust, many dust particles are moved to the airways. Dust particles can be sensed by airways epithelial cells, activate macrophages, dendritic cells and innate immune cells and then initiate responses in various populations of specific immune cells such as T helper cells subsets (Th1, Th2, Th17), T cytotoxic cells and B cells. Initiation of inflammatory immune responses, activation of immune cells and releases of many cytokines, chemokines and other inflammatory molecules, have variable pathologic affects on lung in different respiratory diseases. Unfortunately control of desert dusts is more difficult than control of air pollution. For prevention and treatment of respiratory diseases that are caused by desert dusts, researchers need well-designed epidemiological studies, combined with analysis of the precise composition of sand dusts, and the precise mechanisms of the immune responses. Recognizing the exact cellular and molecular immune mechanisms would be very useful to find new approaches for treatment of desert dust associated pulmonary diseases.
Keywords: Sand dusts, T helper cells, cytokines and chemokines, pulmonary disease
Introduction
Dust storms have an important influence on air quality management because they can have effects on a local, regional and even global scale in the short- and long-term [1-3]. Transport of dust across the Atlantic and Pacific oceans has significantly increased in past years and geological evidence shows that the dust clouds originate predominantly from Africa and Asia [4-6].
The increase in African dust transport to the Americas has adversely affected the health of Caribbean coral reefs and in south Florida almost half of airborne particles in the summer season have originated from Africa [7-9]. Transportation of dusts from North Africa to Southern and Central Europe has also been described [10,11].
Studies have shown that occurrence of dust storm in the Middle East (called the Middle Eastern Dust (MED) storms), is characterized by high concentrations of particles with 2 to 20 μm diameter size, with more than 85% of particles measuring less than 10 μm in diameter [12,13]. According to the study of Leon and Legrand, the major sources of MED storms are Arabian Peninsula, Kuwait, Iraq and parts of Iran [109]. In arid areas of Iraq and Kuwait there are high content of fine particles that are associated or suspected to be associated with respiratory disease, such as desert lung syndrome and severe acute pneumonitis [14-18].
The mineralogical and chemical compositions of dust particles depend on geographic locations. For example the sand in Middle East is mostly composed of silicate minerals, carbonates, oxides, sulfates, and salts in different proportions. In addition recent studies have shown organic nitrogen in rainwater is related to dust originating from Sahara desert dust [19-21]. These small and insoluble particles contain various soluble contaminants in their matrix and on their surface and may also be carriers of anthropogenic pollutants [22-24]. In addition, many microorganisms associated with these dusts can withstand environmental stresses such as high temperatures, ultraviolet (UV) radiation, and atmospheric transport [25].
Various components of dust can access the respiratory system by inhalation and directly affect the epithelium of the human airways [26]. These responses can be exacerbated by biological agents and other trace elements (such as mercury, cadmium, arsenic) that are part of the dust cloud [27]. The exposure to these dust particles and associated contaminants can cause pulmonary diseases that have significant impact on health and quality of life. In many dust induced respiratory diseases, the immune system is playing a dual role. Although activation of the immune system is necessary for removal of dust particles, microorganisms and antigens from the airways; an inappropriate or unchecked immune response can result in severe lung disease and pathologic outcomes (Figure 1). Recently many studies have focused on the suppression of the immune responses for the treatment of asthma and other pulmonary diseases. Likewise, precise tracking of critical immune cells and mediators, such as cytokines, would constitute an important step forward for the management of dust-storm associated pulmonary diseases.
Figure 1.
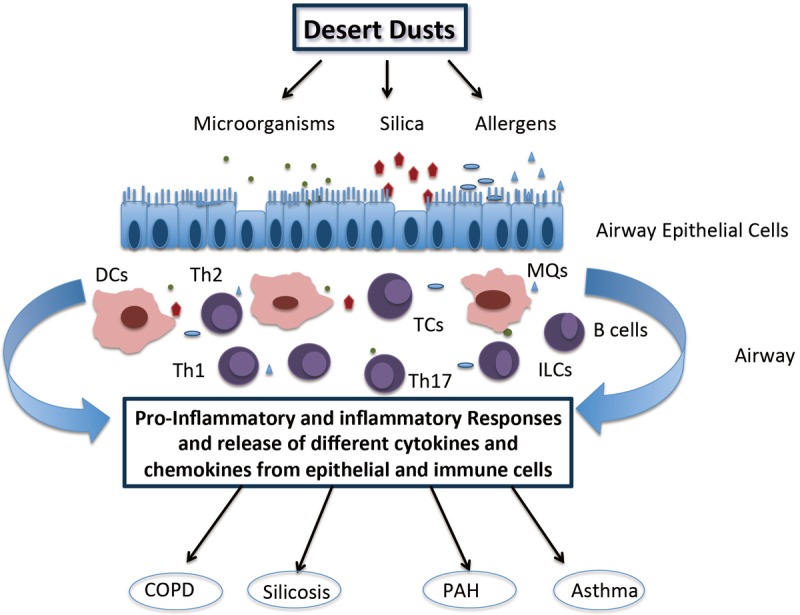
Immune responses to different particles in desert dust. Various components of dust that penetrate into the airways have effects on the epithelium. In addition to the physical barrier role of airway epithelial cells, these cells also play important roles for the immune response. Interacting with airway epithelial cells, macrophages, dendritic cells and innate lymphoid cells are activated and contribute to the inflammatory immune response. Furthermore, cross-talk between epithelial cells and dendritic cells (DCs) can mature the antigen presenting capabilities. DCs can present antigen to different subsets of T helper cells. As result of the cellular interactions, other immune cells such as B cells and T cytotoxic cells can also be activated in response to dust particles in the airways. Finally activation of immune responses and release of various cytokines and chemokines contributes to different pulmonary diseases including asthma, chronic obstructive pulmonary disease (COPD), pulmonary arterial hypertension (PAH) and silicosis. Abbreviations: Dcs = Dendritic cells, MQs = Macrophages, ILCs = Innate lymphoid cells, TCs = T cytotoxic cells, Th1 = T helper cell type 1, Th2 = T helper cell type 2, Th17 = T helper cell type 17.
Asthma, chronic obstructive pulmonary disease (COPD) and desert dusts
The highest prevalence of asthma has been reported in areas with desert dust storm events, such as Middle East [28,29]. Indeed in many parts of the world, sand storms have been linked to asthma exacerbation [30-32]. In the 1980s, Packe and colleagues observed an epidemic of asthma after a thunderstorm [33]. In Athens, Greece, Saharan dust events have been associated with pediatric asthma emergency admittances and in Italy, respiratory mortality has increased among elderly during Saharan dust days [34,35].
In Trinidad, Saharan dusts were linked to pediatric asthma exacerbations and in other parts of world such as East China, the Korean peninsula, Japan and Kuwait, studies have shown a clear association between Asian dust events and respiratory disease particularly asthma [1,36-39].
The incidence of allergic asthma has increased steadily globally. Some of the asthma causing allergens, for example fungal spores, dust mites, plant and grass pollens, anthropogenic emissions, and organic detritus are also found in desert dusts [40-43].
Among these allergens, house dust mites have been a predominant cause of the increase in allergic asthma. Ichinose et al. have shown that Dermatophagoides Farinae transported in Asian sand dust, enhances the incidences of allergic asthma [42,44].
Chronic Obstructive Pulmonary Disease (COPD) can be induced by long-term inhalation of harmful dust particles. In COPD, patients have different respiratory symptoms and systemic outcomes. In general, there is a non-infectious inflammation of the airways and lung parenchyma in COPD [45-48]. The content of chemical elements in rainwater is a useful indicator of airborne dust components and Tubek et al. have found a strong correlation between the concentration of several chemical elements in rainwater and COPD [49].
Mechanism of desert dust mediated exacerbations of inflammatory lung diseases
Airway epithelial cells act as physical barrier in the lung, and also play important roles in the immune response against dust (Figure 1). These cells express different receptors such as Toll-like receptors (TLRs), C-type lectin receptors (CTRs) and protease-activated receptors (PARs) that can be triggered by environmental allergens or microbial components [50]. Following receptor mediated signaling, epithelial cells produce pro-inflammatory cytokines such as IL-6 and IL-8 in response to environmental particles. In addition, these cells release inflammatory mediators such as IL-25, IL-33 and TSLP [51]. This mechanism stimulates lymphocytes, dendritic cells, and granulocytes, including recruitment of neutrophils, resulting in acute inflammation [52-54]. In addition, elaboration of these cytokines initiates and exacerbates Th2 type immune response in asthmatic patients [50,55].
Th2 immune cells are most important cells in progress for allergic asthma and eosinophilic inflammation. After activation of Th2 cells the Th2 cytokine pathway will be triggered; IL-4 and IL-13 are two major cytokines of Th2 cells, assisting in the production of IgE. Among the other cytokines produced by Th2 cells, IL-5 has important roles in the terminal maturation of eosinophils. On the other hand, Interleukin 13 is being involved in mucus production, airway remodeling and fibrosis [56].
In addition to Th2 cells, Th17 cells have important roles in pathogenesis of asthma and allergic airway disease. Th17 cells are a T cell effector subset that produce high levels of IL-17 and IL-22 cytokines [57]. The family of IL-17 cytokines consist of six members (IL-17A, B, C, D, E, F). IL-17E is also known as IL-25 and is an initiator of Th2 responses. Il-17A and IL-17F have important roles in the recruitment, activation, and migration of neutrophils to inflamed sites. These two cytokines also induce production of down-stream cytokines, chemokines and metalloproteinases, all of which are important contributors to inflammation of the Th17 type [58,59].
Innate lymphoid cells 2 (ILC2), another group of innate lymphocyte-like cells, are also involved in inflammation and remodeling in asthma. ILC2 cells resemble Th2 cells, produce IL-4, IL-5, and IL-13 after activation. ILC2 are activated via innate receptors. This represents a different activation pathway when compared to stimulation of Th2 cells. Th2 cells are classical T cells that are activated via the T cell receptor recognizing cognate antigen preferentially presented by dendritic cells. ILC2 cells contribute to airway hyperreactivity and could be important in the initiation of the acute allergic responses [60,61].
The pattern of immune response in COPD is different from asthma. In COPD the innate immune response is perhaps more important than the acquired immune response for the development of persistent and progressive inflammation and remodeling of the lungs. Several studies have shown that T cytotoxic cell mediated immune responses have a critical role in COPD [62-64]. Moreover, Th1 and Th17 cells are also frequently found in COPD [65-70].
Silicosis
Silica (SiO2), which is mainly derived from feldspar and quartz, is the major mineralogical component of Asian sand dusts. Long-term exposure to crystalline silica causes silicosis. Silicosis is a chronic occupational pulmonary disease, which is characterized by inflammation and fibrosis of the lung [71,72]. After inhalation, silica particles are quickly engulfed by alveolar macrophages and in response these cells release inflammatory mediators [73,74]. Excessive exposure to silica also has been associated with tuberculosis, chronic bronchitis, COPD, and lung cancer [75]. McCormic et al. have found that silica can be a risk factor for developing Systemic Sclerosis (SSc) in men and in SSc, pulmonary fibrosis and pulmonary arterial hypertension (PAH) are causes of SSc-related deaths [76].
The immune response polarization in silicosis is not fully understood. In experimental silicosis both Th1 and Th2 cells are associated with the development of silicosis in experimental animals (Figure 1) [77-82]. Moreover, Holian et al. has shown that innate immune responses have a marked and predominant role in the pathogenesis of silicosis in mice [83].
Microorganisms in desert dust and respiratory disease
Different species of pathogenic and non-pathogenic bacteria are constituents of desert dust. However, currently the virulence characteristics of these microorganisms are not well understood and need to be further investigated [84]. However it is clear that the presence of potentially pathogenic microorganisms in respirable particles (≤ 2.5 μm) could contribute to various health effects, especially in the respiratory system. Gwang Pyo. et al. have suggested that different microorganisms are transported in East Asia during Yellow Sand events which is known to be linked to increased incidence of infection which leads to significant adverse health effects [85,86].
It is believed that dust storms can serve as carriers for the pathogens, promoting infections upon inhalation. For example Neisseria meningitides residing in the mucosa can gain access to underlying tissue and blood following exposure to particles from dust storms [87]. Other pathogens have also been detected in dust storm particles. According to a current outline of the current state of affairs in the Middle East, many of the opportunistic dust-borne pathogens can play an important role in human health [25,88-93].
Microbial products including lipopolysaccharide (LPS) that is a glycolipid of gram-negative bacteria cell wall, and β-glucan that is the major constituent of fungi wall are also found in sand dust particles. These microbial components can cause neutrophilic pulmonary inflammation [94,95]. Pathogen-associated molecules of bacteria, viruses, and other pathogens, such as LPS and β-glucan are recognized by pattern recognition receptors (PRRs), for example Toll Like Receptors (TLRs), on epithelial cells, macrophage and dendritic cells in the lungs (Figure 1). Signaling via the PRRs results in the release of different pro-inflammatory cytokines and chemokines, and combined with the induced maturation of the antigen-presenting capacity of dendritic cells can precipitate activation of innate lymphoid cells, T helper cells (Th1, Th2, Th17), T cytotoxic cells and B cells. Hence, in a microbial infection related to variety of antigens, different immune cells would be activated. In the case of dust-storm associated microbial exposures the immune activation could be the cause of pathogenic outcomes in the lungs [96,97].
Pulmonary arterial hypertension and Deseret events
Pulmonary arterial hypertension (PAH) describes a group of pulmonary diseases that are distinguished by remodeling of the small pulmonary arteries and increases in the pressure of the pulmonary circulation and the right ventricle. Furthermore, PAH is a prevalent co-morbidity of COPD, and silicosis [98-100]. Tubek et al. have shown a positive correlation between some chemical elements in rainwater (as an indicator of airborne dust) such as Zinc and Cadmium and arterial hypertension [49].
Different pathogenic mechanisms contribute PAH and recent studies have shown a role for inflammation (Figure 1) [101-106]. Although the exact mechanism of immune response in PAH has not yet determined, specific type 1 and type 2 immune response, and also pathologic roles of Th17 cells have demonstrated [104,107,108].
Conclusion
In conclusion, future research should focus on the pulmonary effects of chronic exposure with desert dust in different parts of the world by well-designed epidemiological studies. This could be made possible by collaborative research programs. In these studies the chemical and biological characterization of ambient particulate matter in urban areas before and after of dust events will be crucial. These data are expected to provide critical information for improving public health.
Although the knowledge of adverse health effects of desert dusts exposure would be useful to prevent deleterious outcomes, for example by establishing an alarm system that would warn the public to avoid outdoor exposures during dust events, the development of therapeutic options for the management of dust storm related respiratory disease is also very important. Today immunotherapy and immunosuppressive therapy methods are used as a treatment approach for many diseases such as allergic asthma. Induction of allergen-specific tolerance by allergen-injection-therapy is a common therapeutic modality, and anti-cytokine therapy is in advanced development with many of preclinical, phase I and II trials planned or currently ongoing for asthma and other respiratory disease. More knowledge about the exact immune mechanisms that elaborated cytokines and other mediators, could lead to an effective treatment for respiratory diseases that are caused by desert dust exposure.
Disclosure of conflict of interest
None.
References
- 1.Husar RB, Tratt DM, Schichtel BA, Falke SR, Li F, Jaffe D, Gasso S, Gill T, Laulainen NS, Lu F, Reheis MC, Chun Y, Westphal D, Holben BN, Gueymard C, McKendry I, Kuring N, Feldman GC, McClain C, Frouin RJ, Merrill J, DuBois D, Vignola F, Murayama T, Nickovic S, Wilson WE, Sassen K, Sugimoto N, Malm WC. Asian dust events of April 1998. J Geophys Res. 2001;106:18317–18330. [Google Scholar]
- 2.Wang Y, Zhuang GS, Tang AH, Zhang WJ, Sun YL, Wang ZF, An ZS. The evolution of chemical components of aerosols at five monitoring sites of China during dust storms. Atmospheric Environment. 2007;41:1091–1106. [Google Scholar]
- 3.Fairlie TD, Jacob DJ, Park RJ. The impact of transpacific transport of mineral dust in the United States. Atmospheric Environment. 2007;41:1251–1266. [Google Scholar]
- 4.Prospero JM, Lamb PJ. African droughts and dust transport to the Caribbean: climate change implications. Science. 2003;302:1024–1027. doi: 10.1126/science.1089915. [DOI] [PubMed] [Google Scholar]
- 5.Prospero JM, Blades E, Naidu R, Mathison G, Thani H, Lavoie MC. Relationship between African dust carried in the Atlantic trade winds and surges in pediatric asthma attendances in the Caribbean. Int J Biometeorol. 2008;52:823–832. doi: 10.1007/s00484-008-0176-1. [DOI] [PubMed] [Google Scholar]
- 6.Middleton NJ. A Geography of Dust Storms in Southwest Asia. Journal of Climatology. 1986;6:183–196. [Google Scholar]
- 7.Garrison VH, Shinn EA, Foreman WT, Griffin DW, Holmes CW, Kellogg CA, Majewski MS, Richardson LL, Ritchie KB, Smith GW. African and Asian dust: from desert soils to coral reefs. Bioscience. 2003;53:469–480. [Google Scholar]
- 8.Shinn EA, Smith GW, Prospero JM, Betzer P, Hayes ML, Garrison V, Barber RT. African dust and the demise of Caribbean coral reefs. Geophysical Research Letters. 2000;27:3029–3032. [Google Scholar]
- 9.Prospero JM. Long-range transport of mineral dust in the global atmosphere: impact of African dust on the environment of the southeastern United States. Proc Natl Acad Sci U S A. 1999;96:3396–3403. doi: 10.1073/pnas.96.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila A, QueraltMitjans I, Alarcon M. Mineralogical composition of African dust delivered by red rains over northeastern Spain. Journal of Geophysical Research: Atmospheres. 1997;102:21977–21996. [Google Scholar]
- 11.Rodriguez S, Querol X, Alastuey A, Viana MM, Mantilla E. Events affecting levels and seasonal evolution of airborne particulate matter concentrations in the Western Mediterranean. Environ Sci Technol. 2003;37:216–222. doi: 10.1021/es020106p. [DOI] [PubMed] [Google Scholar]
- 12.Perdue P, Kazarian KK, Odeyale C, Quance J, Hayward I, Williams T. The surgical significance of Persian Gulf sand. Mil Med. 1992;157:375–377. [PubMed] [Google Scholar]
- 13.Draxler RR, Gillette DA, Kirkpatrick JS, Heller J. Estimating PM10 air concentrations from dust storms in Iraq, Kuwait and Saudi Arabia. Atmospheric Environment. 2001;35:4315–4330. [Google Scholar]
- 14.Hawass ND. An association between ‘desert lung’ and cataract--a new syndrome. Br J Ophthalmol. 1987;71:694–697. doi: 10.1136/bjo.71.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch M, Bar-Ziv J, Lehmann E, Goldberg GM. Simple siliceous pneumoconiosis of Bedouin females in the Negev desert. Clin Radiol. 1974;25:507–510. doi: 10.1016/s0009-9260(74)80135-2. [DOI] [PubMed] [Google Scholar]
- 16.Nouh MS. Is the desert lung syndrome (nonoccupational dust pneumoconiosis) a variant of pulmonary alveolar microlithiasis? Report of 4 cases with review of the literature. Respiration. 1989;55:122–126. doi: 10.1159/000195715. [DOI] [PubMed] [Google Scholar]
- 17.Policard A, Collet A. Deposition of siliceous dust in the lungs of the inhabitants of the Saharan regions. AMA Arch Ind Hyg Occup Med. 1952;5:527–534. [PubMed] [Google Scholar]
- 18.Command UAM, Dis NCI. Severe acute pneumonitis among deployed U. S. military personnel - Southwest Asia, March-August 2003 (Reprinted from MMWR, vol 52, pg 857-859, 2003) Jama-Journal of the American Medical Association. 2003;290:1845–1846. [Google Scholar]
- 19.Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East--Part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21:327–336. doi: 10.1080/08958370802464299. [DOI] [PubMed] [Google Scholar]
- 20.Doganay H, Akcali D, Goktas T, Caglar K, Erbas D, Saydam C, Bolay H. African dust-laden atmospheric conditions activate the trigeminovascular system. Cephalalgia. 2009;29:1059–1068. doi: 10.1111/j.1468-2982.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 21.Mace KA. Organic nitrogen in rain and aerosol in the eastern Mediterranean atmosphere: An association with atmospheric dust. J Geophys Res. 2003;108:11. [Google Scholar]
- 22.Erel Y, Kalderon-Asael B, Dayan U, Sandler A. European atmospheric pollution imported by cooler air masses to the Eastern Mediterranean during the summer. Environ Sci Technol. 2007;41:5198–5203. doi: 10.1021/es062247n. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez S, Alastuey A, Alonso-Perez S, Querol X, Cuevas E, Abreu-Afonso J, Viana M, Perez N, Pandolfi M, de la Rosa J. Transport of desert dust mixed with North African industrial pollutants in the subtropical Saharan Air Layer. Atmospheric Chemistry and Physics. 2011;11:6663–6685. [Google Scholar]
- 24.Querol X, Pey J, Pandolfi M, Alastuey A, Cusack M, Perez N, Moreno T, Viana M, Mihalopoulos N, Kallos G, Kleanthous S. African dust contributions to mean ambient PM10 mass-levels across the Mediterranean Basin. Atmospheric Environment. 2009;43:4266–4277. [Google Scholar]
- 25.Griffin DW. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin Microbiol Rev. 2007;20:459–477. doi: 10.1128/CMR.00039-06. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe M, Igishi T, Burioka N, Yamasaki A, Kurai J, Takeuchi H, Sako T, Yoshida A, Yoneda K, Fukuoka Y, Nakamoto M, Hasegawa Y, Chikumi H, Matsumoto S, Minato S, Horasaki K, Shimizu E. Pollen augments the influence of desert dust on symptoms of adult asthma patients. Allergol Int. 2011;60:517–524. doi: 10.2332/allergolint.10-OA-0298. [DOI] [PubMed] [Google Scholar]
- 27.Cook AG, Weinstein P, Centeno JA. Health effects of natural dust: role of trace elements and compounds. Biol Trace Elem Res. 2005;103:1–15. doi: 10.1385/BTER:103:1:001. [DOI] [PubMed] [Google Scholar]
- 28.Al Frayh AR, Shakoor Z, Gad El Rab MO, Hasnain SM. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001;86:292–296. doi: 10.1016/s1081-1206(10)63301-7. [DOI] [PubMed] [Google Scholar]
- 29.Bener A, Abdulrazzaq YM, Al-Mutawwa J, Debuse P. Genetic and environmental factors associated with asthma. Hum Biol. 1996;68:405–414. [PubMed] [Google Scholar]
- 30.Bellomo R, Gigliotti P, Treloar A, Holmes P, Suphioglu C, Singh MB, Knox B. Two consecutive thunderstorm associated epidemics of asthma in the city of Melbourne. The possible role of rye grass pollen. Med J Aust. 1992;156:834–837. doi: 10.5694/j.1326-5377.1992.tb136994.x. [DOI] [PubMed] [Google Scholar]
- 31.Celenza A, Fothergill J, Kupek E, Shaw RJ. Thunderstorm associated asthma: a detailed analysis of environmental factors. BMJ. 1996;312:604–607. doi: 10.1136/bmj.312.7031.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newson R, Strachan D, Archibald E, Emberlin J, Hardaker P, Collier C. Effect of thunderstorms and airborne grass pollen on the incidence of acute asthma in England, 1990-94. Thorax. 1997;52:680–685. doi: 10.1136/thx.52.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Packe GE, Ayres JG. Asthma outbreak during a thunderstorm. Lancet. 1985;2:199–204. doi: 10.1016/s0140-6736(85)91510-7. [DOI] [PubMed] [Google Scholar]
- 34.Zauli Sajani S, Miglio R, Bonasoni P, Cristofanelli P, Marinoni A, Sartini C, Goldoni CA, De Girolamo G, Lauriola P. Saharan dust and daily mortality in Emilia-Romagna (Italy) Occup Environ Med. 2011;68:446–451. doi: 10.1136/oem.2010.058156. [DOI] [PubMed] [Google Scholar]
- 35.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evi dence of association and effect modification. Environ Res. 2011;111:418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Gyan K, Henry W, Lacaille S, Laloo A, Lamsee-Ebanks C, McKay S, Antoine RM, Monteil MA. African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. Int J Biometeorol. 2005;49:371–376. doi: 10.1007/s00484-005-0257-3. [DOI] [PubMed] [Google Scholar]
- 37.Yoo Y, Choung JT, Yu J, Kim do K, Koh YY. Acute effects of Asian dust events on respiratory symptoms and peak expiratory flow in children with mild asthma. J Korean Med Sci. 2008;23:66–71. doi: 10.3346/jkms.2008.23.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang CY, Tsai SS, Chang CC, Ho SC. Effects of Asian dust storm events on daily admissions for asthma in Taipei, Taiwan. Inhal Toxicol. 2005;17:817–821. doi: 10.1080/08958370500241254. [DOI] [PubMed] [Google Scholar]
- 39.Thalib L, Al-Taiar A. Dust storms and the risk of asthma admissions to hospitals in Kuwait. Sci Total Environ. 2012;433:347–351. doi: 10.1016/j.scitotenv.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 40.Bjorksten B, Dumitrascu D, Foucard T, Khetsuriani N, Khaitov R, Leja M, Lis G, Pekkanen J, Priftanji A, Riikjarv MA. Prevalence of childhood asthma, rhinitis and eczema in Scandinavia and Eastern Europe. Eur Respir J. 1998;12:432–437. doi: 10.1183/09031936.98.12020432. [DOI] [PubMed] [Google Scholar]
- 41.Shamssain MH, Shamsian N. Prevalence and severity of asthma, rhinitis, and atopic eczema in 13- to 14-year-old schoolchildren from the northeast of England. Ann Allergy Asthma Immunol. 2001;86:428–432. doi: 10.1016/S1081-1206(10)62490-8. [DOI] [PubMed] [Google Scholar]
- 42.Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 43.Ezeamuzie CI, Thomson MS, Al-Ali S, Dowaisan A, Khan M, Hijazi Z. Asthma in the desert: spectrum of the sensitizing aeroallergens. Allergy. 2000;55:157–162. doi: 10.1034/j.1398-9995.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 44.Ichinose T, Sadakane K, Takano H, Yanagisawa R, Nishikawa M, Mori I, Kawazato H, Yasuda A, Hiyoshi K, Shibamoto T. Enhancement of mite allergen-induced eosinophil infiltration in the murine airway and local cytokine/chemokine expression by Asian sand dust. J Toxicol Environ Health A. 2006;69:1571–1585. doi: 10.1080/15287390500470833. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, Shimizu K, Betsuyaku T, Ito YM, Fuke S, Igarashi T, Akiyama Y, Ogura S Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 46.Gonem S, Raj V, Wardlaw AJ, Pavord ID, Green R, Siddiqui S. Phenotyping airways disease: an A to E approach. Clin Exp Allergy. 2012;42:1664–1683. doi: 10.1111/j.1365-2222.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 47.Casanova C, de Torres JP, Aguirre-Jaime A, Pinto- Plata V, Marin JM, Cordoba E, Baz R, Cote C, Celli BR. The Progression of Chronic Obstructive Pulmonary Disease Is Heterogeneous The Experience of the BODE Cohort. Am J Respir Crit Care Med. 2011;184:1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 48.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 49.Tubek S, Tubek S. The content of elements in rainwater and its relation to the frequency of hospitalization for arterial hypertension, chronic obstructive pulmonary disease, and psoriasis in Opole Voivodship, Poland during 2000-2002. Biol Trace Elem Res. 2008;123:270–276. doi: 10.1007/s12011-008-8096-9. [DOI] [PubMed] [Google Scholar]
- 50.Wang JY. The innate immune response in house dust mite-induced allergic inflammation. Allergy Asthma Immunol Res. 2013;5:68–74. doi: 10.4168/aair.2013.5.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 52.Thacker EL. Lung inflammatory responses. Vet Res. 2006;37:469–486. doi: 10.1051/vetres:2006011. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 54.Bautista MV, Chen Y, Ivanova VS, Rahimi MK, Watson AM, Rose MC. IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J Immunol. 2009;183:2159–2166. doi: 10.4049/jimmunol.0803022. [DOI] [PubMed] [Google Scholar]
- 55.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013;381:861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 57.Aujla SJ, Alcorn JF. T(H)17 cells in asthma and inflammation. Biochim Biophys Acta. 2011;1810:1066–1079. doi: 10.1016/j.bbagen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 59.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 60.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 61.Hams E, Fallon PG. Innate type 2 cells and asthma. Curr Opin Pharmacol. 2012;12:503–509. doi: 10.1016/j.coph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, Cavallesco G, Tropeano G, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:711–717. doi: 10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 63.Chrysofakis G, Tzanakis N, Kyriakoy D, Tsoumakidou M, Tsiligianni I, Klimathianaki M, Siafakas NM. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125:71–76. doi: 10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- 64.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 65.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Stefano A, Caramori G, Capelli A, Gnemmi I, Ricciardolo FL, Oates T, Donner CF, Chung KF, Barnes PJ, Adcock IM. STAT4 activation in smokers and patients with chronic obstructive pulmonary disease. Eur Respir J. 2004;24:78–85. doi: 10.1183/09031936.04.00080303. [DOI] [PubMed] [Google Scholar]
- 67.Smyth LJ, Starkey C, Vestbo J, Singh D. CD4-regulatory cells in COPD patients. Chest. 2007;132:156–163. doi: 10.1378/chest.07-0083. [DOI] [PubMed] [Google Scholar]
- 68.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 69.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D’Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison OJ, Foley J, Bolognese BJ, Long E 3rd, Podolin PL, Walsh PT. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol Lett. 2008;121:13–21. doi: 10.1016/j.imlet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect. 2000;108(Suppl 4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton RF, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yucesoy B, Vallyathan V, Landsittel DP, Sharp DS, Weston A, Burleson GR, Simeonova P, McKinstry M, Luster MI. Association of tumor necrosis factor-alpha and interleukin-1 gene polymorphisms with silicosis. Toxicol Appl Pharmacol. 2001;172:75–82. doi: 10.1006/taap.2001.9124. [DOI] [PubMed] [Google Scholar]
- 74.Yucesoy B, Vallyathan V, Landsittel DP, Simeonova P, Luster MI. Cytokine polymorphisms in silicosis and other pneumoconioses. Mol Cell Biochem. 2002;234-235:219–224. [PubMed] [Google Scholar]
- 75.Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11:169–173. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- 76.McCormic ZD, Khuder SS, Aryal BK, Ames AL, Khuder SA. Occupational silica exposure as a risk factor for scleroderma: a meta-analysis. Int Arch Occup Environ Health. 2010;83:763–769. doi: 10.1007/s00420-009-0505-7. [DOI] [PubMed] [Google Scholar]
- 77.Davis GS, Pfeiffer LM, Hemenway DR. Expansion of interferon-gamma-producing lung lymphocytes in mouse silicosis. Am J Respir Cell Mol Biol. 1999;20:813–824. doi: 10.1165/ajrcmb.20.4.3407. [DOI] [PubMed] [Google Scholar]
- 78.Davis GS, Pfeiffer LM, Hemenway DR. Interferon-gamma production by specific lung lymphocyte phenotypes in silicosis in mice. Am J Respir Cell Mol Biol. 2000;22:491–501. doi: 10.1165/ajrcmb.22.4.3599. [DOI] [PubMed] [Google Scholar]
- 79.Garn H, Friedetzky A, Davis GS, Hemenway DR, Gemsa D. T-lymphocyte activation in the enlarged thoracic lymph nodes of rats with silicosis. Am J Respir Cell Mol Biol. 1997;16:309–316. doi: 10.1165/ajrcmb.16.3.9070616. [DOI] [PubMed] [Google Scholar]
- 80.Garn H, Friedetzky A, Kirchner A, Jager R, Gemsa D. Experimental silicosis: a shift to a preferential IFN-gamma-based Th1 response in thoracic lymph nodes. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1221–1230. doi: 10.1152/ajplung.2000.278.6.L1221. [DOI] [PubMed] [Google Scholar]
- 81.Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–848. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- 82.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, Vink A, Phan SH, Renauld JC, Lison D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol. 2002;169:2653–2661. doi: 10.4049/jimmunol.169.5.2653. [DOI] [PubMed] [Google Scholar]
- 83.Beamer CA, Migliaccio CT, Jessop F, Trapkus M, Yuan D, Holian A. Innate immune processes are sufficient for driving silicosis in mice. J Leukoc Biol. 2010;88:547–557. doi: 10.1189/jlb.0210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehta SK, Bell-Robinson DM, Groves TO, Stetzenbach LD, Pierson DL. Evaluation of por table air samplers for monitoring airborne culturable bacteria. Aihaj. 2000;61:850–854. doi: 10.1080/15298660008984597. [DOI] [PubMed] [Google Scholar]
- 85.Lee S, Choi B, Yi SM, Ko G. Characterization of microbial community during Asian dust events in Korea. Sci Total Environ. 2009;407:5308–5314. doi: 10.1016/j.scitotenv.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 86.Choi DS, Park YK, Oh SK, Yoon HJ, Kim JC, Seo WJ, Cha SH. Distribution of airborne microorganisms in yellow sands of Korea. J Microbiol. 1997;35:1–9. [Google Scholar]
- 87.Kang HK, Mahan CM, Lee KY, Murphy FM, Simmens SJ, Young HA, Levine PH. Evidence for a deployment-related Gulf War syndrome by factor analysis. Arch Environ Health. 2002;57:61–68. doi: 10.1080/00039890209602918. [DOI] [PubMed] [Google Scholar]
- 88.Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 89.Krause A, Gould FK, Forty J. Prosthetic heart valve endocarditis caused by Bacillus circulans. J Infect. 1999;39:160–162. doi: 10.1016/s0163-4453(99)90011-7. [DOI] [PubMed] [Google Scholar]
- 90.Lee YA, Kim HJ, Lee TW, Kim MJ, Lee MH, Lee JH, Ihm CG. First report of Cryptococcus albidus--induced disseminated cryptococcosis in a renal transplant recipient. Korean J Intern Med. 2004;19:53–57. doi: 10.3904/kjim.2004.19.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim PS, Chen SL, Tsai CY, Pai MA. Pantoea peritonitis in a patient receiving chronic ambulatory peritoneal dialysis. Nephrology (Carlton) 2006;11:97–99. doi: 10.1111/j.1440-1797.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 92.Moissenet D, Bidet P, Garbarg-Chenon A, Arlet G, Vu-Thien H. Ralstonia paucula (Formerly CDC group IV c-2): unsuccessful strain differentiation with PCR-based methods, study of the 16S-23S spacer of the rRNA operon, and comparison with other Ralstonia species (R. eutropha, R. pickettii, R. gilardii, and R. solanacearum) J Clin Microbiol. 2001;39:381–384. doi: 10.1128/JCM.39.1.381-384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park DJ, Yun JC, Baek JE, Jung EY, Lee DW, Kim MA, Chang SH. Relapsing Bacillus licheniformis peritonitis in a continuous ambulatory peritoneal dialysis patient. Nephrology (Carlton) 2006;11:21–22. doi: 10.1111/j.1440-1797.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 94.Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, Morita M. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med. 2002;165:1329–1335. doi: 10.1164/rccm.2108122. [DOI] [PubMed] [Google Scholar]
- 95.Young SH, Robinson VA, Barger M, Porter DW, Frazer DG, Castranova V. Acute inflammation and recovery in rats after intratracheal instillation of a 1-->3-beta-glucan (zymosan A) J Toxicol Environ Health A. 2001;64:311–325. doi: 10.1080/152873901316981303. [DOI] [PubMed] [Google Scholar]
- 96.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 97.Habibzay M, Saldana JI, Goulding J, Lloyd CM, Hussell T. Altered regulation of Toll-like receptor responses impairs antibacterial immunity in the allergic lung. Mucosal Immunol. 2012;5:524–534. doi: 10.1038/mi.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs. 2009;69:1153–1171. doi: 10.2165/00003495-200969090-00002. [DOI] [PubMed] [Google Scholar]
- 99.Mushaben EM, Hershey GK, Pauciulo MW, Nichols WC, Le Cras TD. Chronic allergic inflammation causes vascular remodeling and pulmonary hypertension in BMPR2 hypomorph and wild-type mice. PLoS One. 2012;7:e32468. doi: 10.1371/journal.pone.0032468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monechi G, Fiumalbi C, De Monte MR, Della Scala S, Citroni A, Paolini R, Giannelli M, Melanil C, Barchielli A, Pistolesi P, Bernetti E, Paghi M, Valerio M, Sagramoni , Guerri M, Cannarozzo G, Canocchi A. [Investigation on health status of silica exposed workers in “cotto Fiorentino” companies] . G Ital Med Lav Ergon. 2007;29:736–737. [PubMed] [Google Scholar]
- 101.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–1538. doi: 10.1016/j.jacc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 102.Austin ED, Rock MT, Mosse CA, Vnencak-Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med. 2010;104:454–462. doi: 10.1016/j.rmed.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 104.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park SH, Chen WC, Hoffman C, Marsh LM, West J, Grunig G. Modification of hemodynamic and immune responses to exposure with a weak antigen by the expression of a hypomorphic BMPR2 gene. PLoS One. 2013;8:e55180. doi: 10.1371/journal.pone.0055180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grunig G, Marsh L, Esmaeil N, Jackson K, Gordon T, Reibman J, Kwapiszewska G, Park SH. Perspective. Ambient air pollution: inflammatory response and effects on the lung’1(1):s vasculature. Pulmonary circulation. 2013 doi: 10.1086/674902. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ross DJ, Strieter RM, Fishbein MC, Ardehali A, Belperio JA. Type I immune response cytokine-chemokine cascade is associated with pulmonary arterial hypertension. J Heart Lung Transplant. 2012;31:865–873. doi: 10.1016/j.healun.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 108.Hsu E, Shi HW, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung Tissues in Patients With Systemic Sclerosis Have Gene Expression Patterns Unique to Pulmonary Fibrosis and Pulmonary Hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Léon JF, Legrand M. Mineral dust sources in the surroundings of the north Indian Ocean. Geophys Res Lett. 2013;30:1309–1312. [Google Scholar]


