Abstract
Multiple sclerosis (MS) is a chronic demyelinating immune-mediated disease of the central nervous system. Infections have been implicated in different aspects of the disease such as induction of relapses and possibly, progression. Bladder dysfunction and associated urinary tract colonization (UTC) and infections (UTIs) are common in MS patients. UTIs can exacerbate neurological symptoms in MS, whilst high-dose steroid treatment of acute neurological worsening with concurrent untreated UTC may lead to unmasking of infection. This clinical audit was designed to investigate whether our institution is adhering to the National Institute for Health Care and Excellence (NICE) Clinical Guideline 148 for the management of patients with lower urinary tract symptoms due to neurogenic bladder dysfunction. We identified 21 patients with abnormal urine dipsticks out of 118 patients presenting at Nottingham University Hospitals for clinical review or for assessment of a relapse. Patients were asked about catheter status and the presence of any lower urinary tract symptoms. In all cases of relapse assessment, current practice at our institution had been to delay treatment with methylprednisolone (MP), pending the results of microbiology culture and sensitivity testing. If the patient was confirmed to have an infection, treatment with MP was delayed further awaiting completion of a course of antibiotics. We suggest that corticosteroid treatment need not be delayed but rather administered simultaneously with antibiotic treatment for the UTI, provided that the patient has no systemic symptoms of infection (e.g. fever, rigors, raised CRP). Patients must be educated and cautioned to contact their doctor in the event that systemic symptoms do develop during treatment.
Keywords: Multiple sclerosis, urinary tract infection, relapse, corticosteroids, antibiotic treatment, diagnosis, audit
Introduction
Multiple Sclerosis (MS) is a progressive, immune-mediated inflammatory demyelinating disease of the central nervous system affecting over 100,000 people in the United Kingdom [1,2]. The disease affects three times as many women as men and most people are diagnosed between 20-40 years of age. Relapsing-remitting MS (RRMS) is the most common form of the disease, comprising approximately 85% of all cases. In RRMS, acute relapses are unpredictable in onset and duration, often causing significant disability by sudden impairment of sensory, motor, visual, or other neurological functions. There can be almost complete recovery of function following these episodes; however, disease progression can occur by sustained long-term neurological deficit and loss of function after each relapse. Studies have shown that greater relapse frequency in the early stages of MS may contribute to a worse long-term prognosis as not only partially reversible inflammatory demyelination, but also irreversible axonal loss occurs with each relapse [2,3]. RRMS often progresses to secondary progressive MS (SPMS), where there is a gradual deterioration in function over time independent of relapses [1]. 10-15% of MS patients have a steady worsening of symptoms and increase in disability without relapses or remission (primary progressive MS, PPMS).
MS is a multi-factorial, immune-mediated disease. Genetic and population studies suggest that environmental triggers may initiate the dis ease process in genetically susceptible individuals [4]. Mechanisms of immunopathogenesis are incompletely understood and have been reviewed elsewhere [5].
Infections and MS
Infections are thought to be involved in MS pathogenesis and may influence disease susceptibility and clinical course [6]. Acute relapses may be induced or exacerbated by infection, most commonly in the form of an upper respiratory tract infection (URTI) or urinary tract infection (UTI). Sibley et al [4] was the first to define an at-risk-period of two weeks preceding and five weeks after the onset of an infection, during which the risk of developing a disease relapse is increased, possibly due to enhanced immune activity induced by the infection [2,3]. In substantial agreement with this seminal observation, other investigators also found that exacerbations of disease activity during the at-risk-period were more likely [7,8] and led to more severe and sustained relapses [2]. Correale et al. [9] also studied the role of UTIs and demonstrated an increased risk of relapses during systemic infections, paralled by MRI activity. They also demonstrated in transwell co-cultures of immune cells that the proliferation of antigen-specific T-cell lines in response to myelin antigens was increased during relapses, possibly through bystander, non antigen-specific immune mechanisms. In addition to bystander activation of myelin-specific, autoreactive T cells, other mechanisms including molecular mimicry, superantigen-driven immune cell activation, and direct microbial effects on the CNS have been proposed to explain the observed association between infections and MS [10,11]. Toll-like receptors (TLRs) are key components of the innate immune system. Upon recognition of microbial epitopes, they lead to the production of inflammatory cytokines and interferons which, in turn, drive the development of more specific, adaptive immune responses. We and others have reported that TLRs, in particular TLR2 and its heterodimeric partners TLR1 and TLR6, are expressed on human naïve Tregs, which are defective in MS, and can inhibit their regulatory function [11-13]. The mechanisms mediating the effects of infections on MS clinical exacerbations and possibly, disease progression, remain incompletely understood.
Bladder dysfunction in MS
Bladder dysfunction is common in the majority of patients with MS, causing significant morbidity with adverse effects on quality of life. Detrusor hyperreflexia presents as urinary frequency, urgency and incontinence. Occurring in 50-90% of patients with MS, this is due to an inability to inhibit detrusor contractions, resulting in voiding at low bladder volumes [14]. Half of the patients with detrusor hyperreflexia develop detrusor-sphincter dyssynergia, in which there is a failure of urethral sphincter relaxation on detrusor contraction, resulting in high micturition pressures and symptoms of hesitancy, incomplete emptying, intermittent stream and dysuria. Detrusor areflexia is most commonly due to sacral cord lesions. It presents in approximately 20% of patients as urgency, frequency and urinary retention [14,15].
With progressive disease activity, bladder dysfunction tends to worsen and patients often require pelvic floor exercises, intermittent self-catheterisation (ISC), or an indwelling permanent catheter to improve the management of bladder dysfunction [14,15]. Due to urinary stasis or the use of a catheter, these patients are increasingly susceptible to recurrent UTIs, which may, if left untreated, lead to systemic infection and sepsis. Infection-associated pyrexia may precipitate the neurological deficit by altering the conduction properties of demyelinated axons (Uhtoff’s phenomenon) [16]. Since recurrent UTIs have been proven to be associated with exacerbation of disease activity, their appropriate management in MS patients is essential [1,3,7,9].
Our current practice: diagnosis of UTI during the assessment of a presumed MS relapse
Current practice at Nottingham University Hospitals and many other institutions in the UK is that all patients attending for the assessment of an acute relapse in MS must have a urine dipstick to assess for the presence of a concurrent UTI [17]. The standard treatment for an acute, disabling MS relapse is high-dose corticosteroid therapy, typically either orally for 5 days or intravenously for 3 days [18]. At the time of assessment for a relapse, if urine dipstick is positive for blood, protein, nitrites or leucocyte esterase, and/or the clinical suspicion of UTI is high, the sample is sent for flow cytometry analysis and culture at the hospital microbiology laboratory. Flow cytometry quantifies the presence and concentration of erythrocytes, white blood cells, epithelial cells and bacteria in the sample. If an elevated number of bacteria is identified (>8,040 bacteria/μl at our institution), a culture is performed to determine the bacterial species. Steroid treatment is delayed until the results are available, usually within 48 hours. The decision to send for urinalysis and culture is based on the clinical judgment of the physician at the time of assessment. Where cultured samples yield a single microbial species, patients are treated with antibiotics before commencing steroid treatment. Current practice considers cultures yielding mixed growth as not pathological, but rather contaminated and therefore, not clinically significant. Many patients with urine culture yielding mixed bacterial growth are intermittently self-catheterising or are permanently catheterized. Microbial resistance to antibiotics is identified through antibiotic sensitivity testing, provided a single species has been identified in culture. If a patient is asymptomatic for UTI, antibiotic therapy is withheld awaiting the results of culture and sensitivity. However, if the patient is symptomatic, antibiotics are given empirically and altered if necessary once the results of sensitivity are available. Only once the patient’s concurrent infection has been treated is steroid therapy initiated (See Figure 1).
Figure 1.
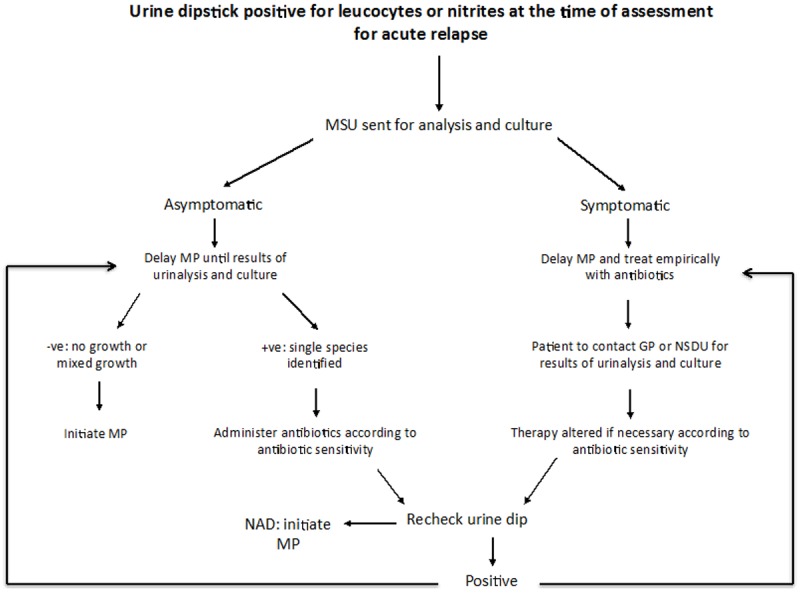
Urine dipstick positive for leucocytes and nitrites at the time of assessment for acute relapse: Algorithm showing current management of the MS relapse patient when there is evidence of urinary tract infection on dipstick analysis.
Clinical audit
Aims and objectives
National Institute for Health Care and Excellence (NICE) guidelines 148 for the management of UTIs in patients with neurogenic bladder dysfunction state that if the dipstick results and person’s symptoms suggest an infection, then clinicians should arrange a urine bacterial culture and antibiotic sensitivity test before starting antibiotic treatment. However, treatment need not be delayed, but may be adapted when results are available [19].
The purpose of this clinical audit was to identify whether our institution is adhering to the NICE guidance protocol, and to identify the types of bacteria most commonly isolated from our patient population and antibiotic sensitivities.
Methods
A total of 118 MS patients who presented to the neurological day unit for the assessment of an acute relapse or to the MS clinic for a neurology review were screened for the presence of a UTI. These patients attended Nottingham University Hospitals between the months of April and August 2013. Patients presenting to the clinic are not routinely screened, however urine samples were obtained for the purposes of the audit after verbal consent. Patient information, details of assessment and infection were collected through discharge letters, patient notes and microbiology results, both current and historical, on hospital electronic records.
Patients were asked about the presence of systemic and bladder symptoms. Systemic symptoms were classified as pyrexia, renal colic or rigors. Bladder symptoms were classified as frequency, urgency, incontinence, retention or dysuria. Patients were also classified according to catheter status (no catheter, permanent, suprapubic, intermittent self-catheter, or un- known).
Results
Patient characteristics
Of the 118 patients that underwent urine dipstick analysis, 21 who tested positive for any of nitrites, leucocytes, blood or protein were included in the clinical audit. For dipstick-positive patients, the median age at diagnosis was 35 years, two-thirds of the patients were female, 13 did not have a catheter in place and just under half had the SPMS subtype of the disease (See Table 1 for patient demographics). Eight of the 21 patients included in the audit attended for review appointments at MS clinic, the remaining 13 attended on the neurospinal day unit for relapse assessment.
Table 1.
Patient Demographicsa
| Gender | |
| M | 7 |
| F | 14 |
| Current age | |
| Mean | 46.3 |
| Median | 45 |
| Min | 25 |
| Max | 71 |
| Catheter | |
| None | 13 |
| ISC | 5 |
| PC | 2 |
| SPC | 1 |
| MS Subtype | |
| RRMS | 8 |
| SPMS | 10 |
| PPMS | 3 |
Patient demographics.
M: male; F: female; ISC: intermittent self-catheterisation; PC: permanent catheter; SPC: suprapubic catheter. The median age at diagnosis was 35 years. 8 of the 21 patients included in the audit attended for review appointments at MS clinic, the remaining 13 attended on the neurospinal day unit for relapse assessment.
Clinical data
Twenty samples were sent for detailed urinalysis and culture. Culture was performed on 18 samples due to elevated levels of bacteria: 6 samples showed no significant growth (defined by the microbiology laboratory at our institution as <105 colony forming units per ml), 1 showed mixed growth and 11 showed a specific bacterial species (see Table 2 and Figure 2). Present in over one-third of patients, urgency was the most frequently reported symptom of lower urinary tract dysfunction. Frequency, incontinence and retention were also commonly reported (see Figure 3). Two-thirds of the patients had no documentation of previous UTIs over the last three years on the hospital patient database (see Figure 4). However, given that UTIs are usually managed by general practitioners (GPs), who may treat empirically with antibiotics, this does not mean that the patients have not had any UTIs in the past. Out of the 7 patients with a documented history of UTIs, 2 were on antibiotic prophylaxis.
Table 2.
Results of urinalysis and culture for dipstick-positive patientsa
| Patient | Dipstick results | MSU | Culture | Catheter status | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| WBC (0-40/uL) | RBC (0-44/uL) | Epithelial cells (0-55/uL) | Bacteria (0-8040/uL) | ||||
| 1 | Positive: leuc, nit, protein, blood | 70 | 10 | 0 | Unknown | No sig growth | SPC |
| 2 | Positive: protein, blood, malodour | Unknown | Unknown | Unknown | Unknown | No sig growth | NC |
| 3 | Positive: nit | 44 | 49 | 9 | 4812 | E. Coli | ISC |
| 4 | Positive: nit, protein, blood | Unknown | Unknown | Unknown | Unknown | No sig growth | NC |
| 5∞ | Positive | 53 | 1 | 14 | 3259 | Citrobacter sp | NC |
| 6 | Positive: leuc, nit | 112 | 2 | 2 | 18977 | E. Coli | ISC |
| 7◊ | Positive: leuc | Not sent | Not sent | Not sent | Not sent | Not sent | ISC |
| 8* | Positive: blood, protein | 69 | 6 | 21 | 12834 | E. Coli | NC |
| 9♠ | Positive: leuc, blood | 40 | 16 | 33 | 2397 | Not performed | NC |
| 10 | Positive: nit | 11 | 3 | 4 | 43362 | E. Coli | ISC |
| 11∞ | Positive | 0 | 0 | 0 | 0 | No sig growth | NC |
| 12 | Positive: leuc | 15 | 4 | 8 | 29216 | E. Coli | NC |
| 13 | Positive: leuc, blood | 321 | 4 | 160 | 2867 | No sig growth | NC |
| 14 | Positive: nit | 376 | 15 | 1 | 82119 | E. Coli | ISC |
| 15 | Positive: nit | 33 | 25 | 44 | 84802 | E. Coli | NC |
| 16 | Positive: leuc | 216 | 4 | 58 | 1744 | No sig growth | NC |
| 17 | Positive: leuc, nit | 2877 | 8 | 48 | 22702 | Mixed growth | PC |
| 18 | Positive: leuc | 143 | 8 | 16 | 303 | B haemolytic strep group B | NC |
| 19♠ | Positive: leuc | 93 | 9 | 34 | 1212 | Not performed | NC |
| 20∞ | Positive | 544 | 582 | 4 | 45046 | E. Coli | PC |
| 21∞ | Positive | Unknown | Unknown | Unknown | Unknown | Coliform | NC |
Symbol legends:
denotes samples where an abnormality was identified on urine dipstick (i.e. positive).
However, documentation in the patient’s notes and discharge letter failed to specify what the urine dipstick was positive for.
denotes a sample where there was failure to send to microbiology for flow cytometry analysis and culture despite the urine dipstick being positive for leucocyte esterase.
denotes a false negative sample where urine dipstick was negative for infection markers, however flow cytometry showed elevated levels of bacteria and culture yielded E. Coli.
denotes samples where elevated levels of white blood cells were found on flow cytometry analysis.
However, as bacterial levels were within the normal range, culture was not performed.
Figure 2.
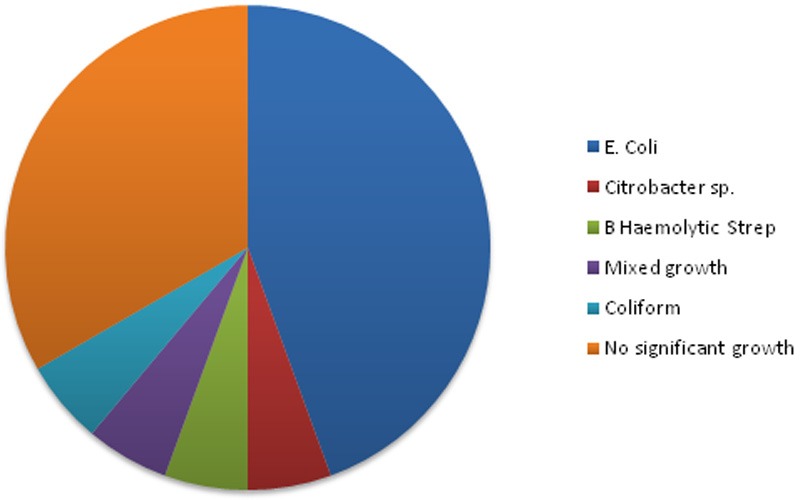
Eighteen samples were cultured: 11 identified a single bacterial species, 1 yielded mixed growth and 6 showed no significant growth. Eight samples yielded E. Coli.
Figure 3.
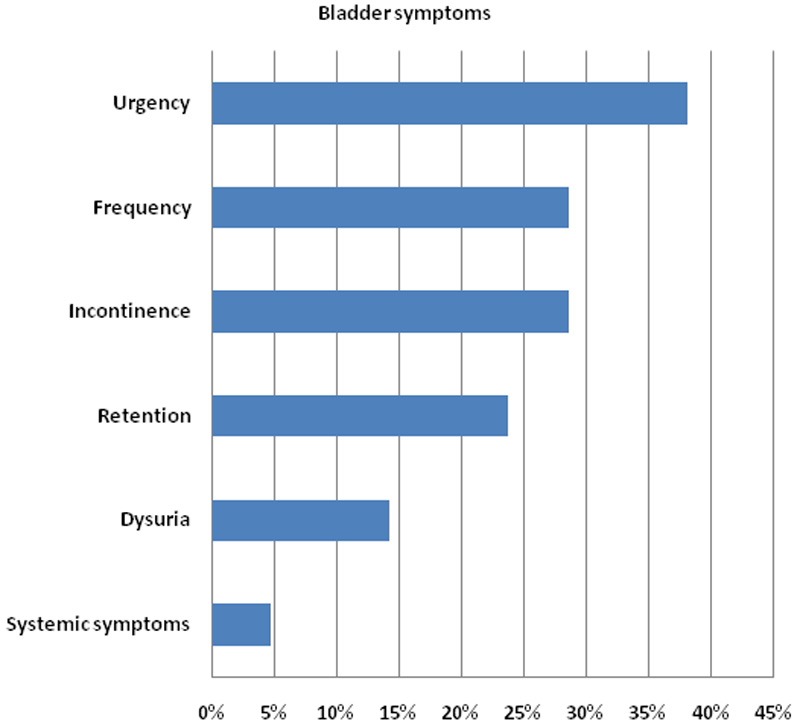
Bladder symptoms: 12 of the 21 patients that had an abnormal urine dipstick confirmed the presence of one or more of the six bladder symptoms included in the audit ProForma. 9 patients expressed no bladder symptoms.
Figure 4.
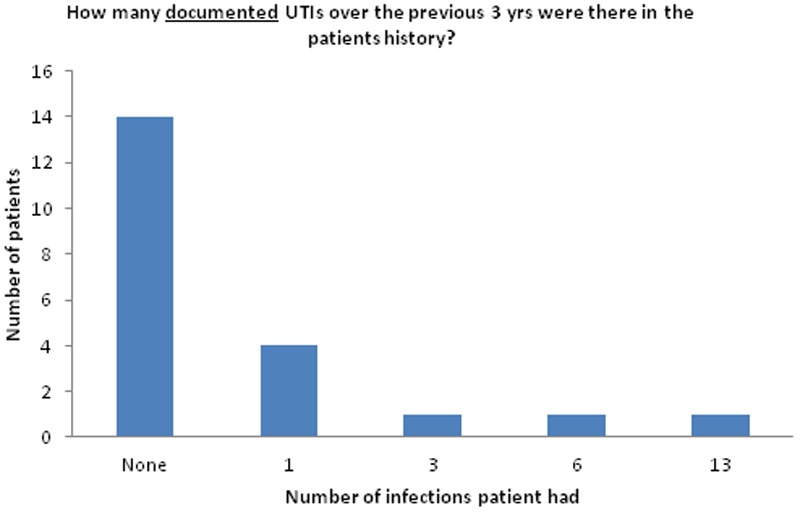
History of UTIs: There were 7 patients that had a documented history of UTIs over the past 3 years. It is important to note that “None” indicated no UTIs were documented in hospital records for these patients. However, given that UTIs are usually managed by GPs, who may treat with empirical antibiotics, this does not mean that the patient has not had any UTIs in the past. Out of the 7 patients with a documented history of UTIs, 2 were on antibiotic prophylaxis.
In three samples the urine dipstick was documented as positive, however there was no documentation of the specific abnormality identified on urine dipstick. One sample was not sent to microbiology despite the urine dipstick identifying leucocyte esterase in the sample (see Table 2). Therefore, flow cytometry analysis and culture were not performed. Furthermore, this patient was not treated empirically for infection. One sample showed evidence of a false negative on urine dipstick analysis which failed to identify any markers for infection. However, flow cytometry analysis and culture yielded elevated levels of bacteria and E. coli respectively. Two samples showed significant pyuria (elevated white blood cells) on flow cytometry analysis; however, the samples were not cultured as the bacterial levels were within the normal range (see Table 2).
Use of antibiotic therapy
Antibiotics were withheld awaiting culture and sensitivity testing in 13 patients. Eight of these patients were confirmed to have a UTI on culture, of which 5 were later prescribed antibiotics according to sensitivity testing. 7 patients received empirical antibiotic therapy, of which 3 returned a significant positive culture. One patient was lost to follow-up.
Discussion
Corticosteroid therapy is contraindicated in the presence of an infection as the immunosuppressive effects of steroids may prolong the course of infection and increase the risk of advancement to systemic infection and potential life-threatening complications [18,20]. Therefore, current practice is to determine the presence of infection prior to the initiation of treatment in an acute relapse. This is very much dependent upon clinical judgment and is achieved through detailed history taking, examination and bedside tests, including the urine dipstick. Urine dipsticks are a quick and efficient screening tool as opposed to the more time consuming detailed urinalysis and culture, which takes 24-48 hours to yield results [17]. This is advantageous in the clinical setting where early steroid treatment is important in order to achieve remission of the disabling symptoms associated with an acute relapse, thereby minimising short-term morbidity, including persistent sensory or motor dysfunction.
When a patient tests positive for infection markers on urine dipstick and is confirmed to have an acute relapse, the physician must determine whether there are any signs of systemic illness. If none are present and the patient is otherwise well, with no significant co-morbidities (i.e. poorly controlled diabetes), we recommend that the clinician need not delay steroid treatment pending the results of flow cytometry and culture. Rather, it would be prudent to minimize patient upset caused by the sudden worsening or new onset of neurological dysfunction and simultaneously treat both the relapse with corticosteroids and the UTI with antibiotics [18]. This could lead to lower levels of patient distress, higher satisfaction rates, lower severity and shorter durations of relapse. To ensure safety is a priority, the patient must be educated and cautioned to contact their physician or return to the hospital if they notice the development of any systemic symptoms (i.e. fever, rigors).
The urine dipstick
Urine dipsticks have specificity and sensitivity values of 70-87% and 68-88% respectively for infection, provided that the sample is positive for both nitrites and leucocyte esterase [18]. Given these values, it is inevitable that a proportion of dipsticks are false negative for infection and that these patients are not followed up through detailed urinalysis and culture and, therefore, not treated for infection.
Furthermore, studies have found leucocytes show significant degradation in the hours after a urine sample is collected. Although boric acid, used as a preservative, reduces sample degradation, one study found that at 4 hours post-collection cell loss was still at 40%, compared with 60% without the use of preservative [21]. This indicates that a number of low-grade infections may be going undetected and therefore, untreated. The time between sample collection and detailed urinary analysis may well exceed four hours, especially in busy hospital laboratories. Considering the cell loss that can occur in this time, the upper threshold of ‘normal’ for WBCs (40/μL), RBCs (44/μL) and epithelial cells (55/μL) may be too high. In particular, the pathology laboratory at our institution and many others currently defines the upper limit of normal for WBCs to be 40 WBC/μL. In cases where WBC levels may be in the upper ranges of normal or just above normal, degradation of the sample at the time of analysis may result in a failure to culture and identify an infection. Consequently, the patient will not be followed up, investigated further and treated for their infection. Some studies define 10 WBC/μL as pathological and treat with antimicrobial therapy accordingly. In a strict sense, pyuria is always pathological and such thresholds are inevitably arbitrary [21,22].
Unfortunately, there is currently no viable alternative available to rival the speed and efficiency of the urine dipstick in identifying a potential infection. A potential solution to this problem is immediate microscopic analysis of fresh urine samples provided by patients presenting for review as an outpatient or for assessment of a relapse as an inpatient. However, this is a time-consuming and expensive task that is only suited to highly specialized research laboratories [22] but not feasible in hospital practice.
Culture
The pathology laboratory at our institution and many others currently defines an infection as a minimum of 105 colony forming units per milliliter (cfu/ml). It should be noted that this widely accepted threshold is based on the work of Kass et al. who sampled acutely unwell women with acute pyelonephritis [23]. Stamm et al. questioned the validity of this threshold in their research and sampled patients with symptoms of frequency and dysuria [24,25]. Their study found that a threshold of 105 cfu/ml was missing more than 50% of UTIs and proposed that a more accurate threshold be 102 cfu/ml [25].
The significance of polymicrobial culture
The majority of urine samples from catheterized patients yield a polymicrobial culture. According to standard protocol, this is often dismissed as contaminated, and therefore, unreliable and clinically insignificant. However, Khasriya et al. argued that there is no evidence to disprove polymicrobial culture as clinically significant [22]. Using spun urinary sediment from MSU samples, they found that in many patients with lower urinary tract symptoms, the urothelium was colonized by multiple bacterial species, including E. coli. This would be expected to influence the treatment regimen, as sensitivity testing on each species may reveal that a single antibiotic therapy is inadequate for the eradication of infection [22]. In addition, in catheterised patients it may be particularly difficult to distinguish between symptoms of UTI and neurogenic bladder. As a result, clinical judgment prevails when taking the decision to treat with antibiotics.
High index of suspicion in clinic
Eight of the twenty-one positive urine dipsticks came from review appointments at the MS clinic. Many of these patients suffer from SPMS and have a higher degree of disability associated with their disease. Although they are not suffering acute relapse symptoms, chronic bladder dysfunction and the need for intermittent or permanent catheterization predispose these patients to recurrent UTIs. They must be asked about the presence of any bladder symptoms, particularly new onset or worsening of existing dysfunction. Whether chronic low-grade infection causes a subclinical increase in disease activity levels and influences disease progression is a question that has been raised but not conclusively answered by a number of researchers [22].
Antibiotic prophylaxis
Two patients were on antibiotic prophylaxis for recurrent UTIs. NICE guidelines state that antibiotic prophylaxis should not routinely be used in patients with neurogenic bladder dysfunction. However, given the nature of bladder symptoms in MS and the propensity for these patients to develop UTIs, it is likely that numerous patients are given long-term antibiotics for chronic low-grade, yet symptomatic, infections. This is not regarded as prophylaxis because the patient has evidence of infection, usually microscopic pyuria of ≥10 WBC/μL. Extended treatment allows for the attenuation of bladder symptoms and therefore better quality of life. Unfortunately, it also limits the number of patients in which a causative bacterial species can be identified and furthermore, it may hinder the evaluation of a relationship between infections and acute relapses or the progression of disease. Interestingly, in our cohort antibiotic sensitivity testing in patients suffering recurrent UTIs often showed the evolution of the pathogen, usually E. coli, in developing resistance to the primary antibiotics, particularly trimethoprim and amoxicillin.
Pseudo-relapses and UTIs
A number of MS patients presenting on the Day Unit for assessment were identified as having a non-disabling relapse, in that they presented with mild sensory dysfunction. In these patients, it was thought steroid treatment was not warranted and they were discharged home. Interestingly, a number of discharge letters reviewed during the collection of data failed to clarify whether or not a urine dipstick had been performed, therefore, lacking information concerning the possibility of an infection-associated relapse. “Pseudorelapses” can present with infective symptoms such as a low-grade fever, but also during exercise or other activities that cause an increase in core body temperature (Uhthoff’s phenomenon) [16]. A relapse is defined as the subacute or acute onset or worsening, over days or weeks, of neurological deficit, (e.g., sensory, motor, visual, etc.) in a patient with established MS, lasting at least 24 h [16]. Uhthoff’s phenomena are usually of shorter duration than immune-mediated relapses and occur in the setting of ion channel modifications in the demyelinated axon, which is known to be very sensitive to even small increases in temperature [16,26]. Potent steroids may not be warranted in cases of mild symptoms in consideration of potential side effects, even if it is clear that neurological deficits are caused by a relapse [27]. Furthermore, as mentioned above steroids are generally contraindicated during infections. Therefore, although the definition of an acute relapse may encompass a variety of presentations, the treatment should be tailored to the patient [18]. The clinical history is very important in establishing whether a neurological deficit can be attributed to Uhthoff’s phenomena or to a genuine relapse [16,26].
Conclusions and recommendations
We conclude from our audit that we are largely compliant with NICE guidance 148 when UTIs are detected in patients with MS. However, we uncovered instances in which UTIs may be under-detected, in particular when patients are asymptomatic, but urine dipstick analysis is positive for either nitrites or leucocytes. In such cases, the literature indicates that UTIs may be present even when automated cultures are considered negative according to hospital standards (<8040 bacteria/μL) or when mixed cultures are detected. The latter finding is routinely considered suggestive of contamination by commensal bacteria, but in fact does not exclude that one of the cultured species (e.g., E coli) may be causing an asymptomatic, but clinically significant infection. We recommend the following:
• If the urine dipstick is abnormal and the patient asymptomatic from the UTI, but judged to be in clinical exacerbation from MS, treatment with high-dose steroid treatment can be given if warranted; a urine sample should be sent for complete analysis and antibiotic treatment started on the basis of bacterial sensitivity if an infection is confirmed when the results become available. The patient must be made aware of the signs of systemic illness and be told to contact their doctor if any of these develop.
• If the patient is symptomatic from the UTI, and judged to be in clinical exacerbation from MS, empirical treatment with antibiotics should be given without delaying high-dose steroid treatment of the clinical relapse if this is warranted; a urine sample should be sent for complete analysis based on clinical judgment, even if the dipstick is normal. Antibiotic treatment should be either confirmed or modified on the basis of bacterial sensitivity when the results become available.
• If the patient is symptomatic from the UTI and systemically ill, empirical treatment with antibiotics should be given but steroid treatment should be withheld to prevent septic complications.
• The urine dipstick may be missing a large number of low-grade infections. Although it is advisable to dipstick all patients that attend for the assessment of an acute relapse, given the relatively low sensitivity and specificity values, it is prudent that clinical judgment prevail when considering a concurrent UTI. Where the dipstick is negative for infection but the patient confirms new lower urinary tract symptoms or a worsening of existing bladder dysfunction on questioning, it would be sensible to send a urine sample to microbiology and treat with empirical antibiotic therapy.
• It is important that all MS patients are questioned for the new onset or worsening of lower urinary tract symptoms. Clinicians should have a high suspicion for UTI. In the setting of MS outpatient clinics, on the basis of our finding of asymptomatic bacterial colonisation, we recommend to routinely perform urine dipstick test when practical, followed by MSU analysis where abnormalities are identified.
• In catheterized patients, a polymicrobial culture must not be dismissed as clinically insignificant too hastily as the catheter provides a niche for the colonization of the urinary tract by multiple microbes. In these patients, one of these microbial species (most commonly E. coli), may well be acting as a pathogen in the presence of other commensal organisms [22].
Opportunity for further research
A much greater number of patients than usually detected could be suffering from covert infection and are, currently, going unidentified and untreated. Bladder irritation caused by urinary stasis in patients with neurogenic bladder, may result in chronic microscopic pyuria and low-grade infection. Bacterial levels may not be elevated above normal on detailed urinalysis. However, epithelial cells, red blood cells, protein and white blood cells can be elevated or in the upper ranges of normal. Moreover, it has recently been shown that uroepithelial cells may harbor intracellular pathogens, which may be responsible for low-grade infection that is difficult to treat with antibiotic therapy. Remarkably, such intracellular pathogens may be quite distinct from those most commonly identified in urinary suspension [22].
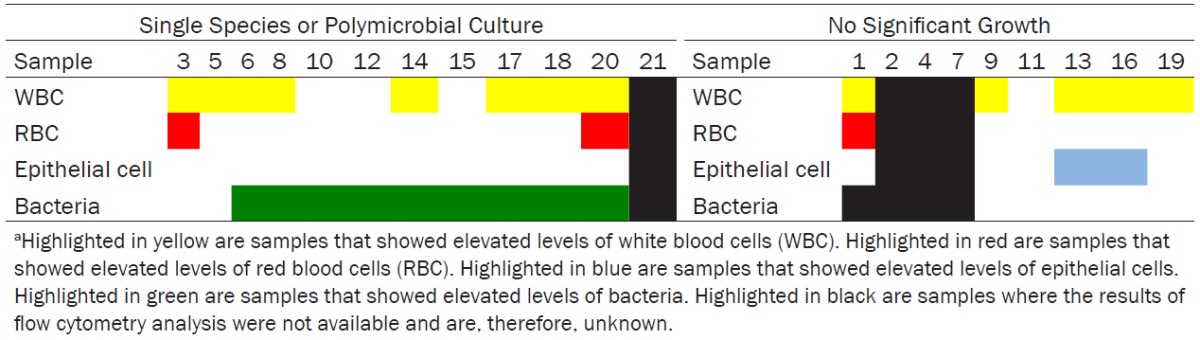
Consistent with this concept, our audit showed that the majority of samples that yielded a single microbe or polymicrobial flora had elevated levels of bacteria on flow cytometry analysis. In the case of samples that yielded no significant growth, the levels of bacteria were not elevated by laboratory standards of 8040/μL, however many of the patients had significant pyuria. We hypothesize that the reason for pyuria may be intracellular bacterial infection, which cannot be identified on flow cytometry or by current culture methods. This may be particularly true for the samples where elevated levels of epithelial cells were identified (See Table 3). Chronic immune activity due to covert or low grade infection may have an effect on disease activity in MS, which has yet to be investigated.
Table 3.
Abnormal Cells vs. Significant Growtha
|
Acknowledgements
We thank Professor Cris S. Constantinescu for critical reading of the manuscript.
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry. 2014;85:76–84. doi: 10.1136/jnnp-2013-305450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lublin FD, Cutter GR, Baier M. Exacerbation recovery and the progression of Multiple Sclerosis. Neurology. 2000;54:A216–A217. [Google Scholar]
- 4.Sibley WA, Bamford CR, Clark K. Clinical Viral-Infections and Multiple-Sclerosis. Lancet. 1985;1:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat R, Steinman L. Innate and Adaptive Autoimmunity Directed to the Central Nervous System. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 7.Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64:736–741. doi: 10.1136/jnnp.64.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, van Der Meché FG, van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- 9.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- 10.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 11.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but Direct Abolishment of Human Regulatory T Cell Suppressive Capacity by Various TLR2 Ligands. J Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- 13.Nyirenda MH, O’Brien K, Sanvito L, Constantinescu CS, Gran B. Modulation of regulatory T cells in health and disease: role of toll-like receptors. Inflamm Allergy Drug Targets. 2009;8:124–129. doi: 10.2174/187152809788462581. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas R, Young C, Friede T. Bladder symptoms in multiple sclerosis: a review of pathophysiology and management. Expert Opin Drug Saf. 2010;9:905–915. doi: 10.1517/14740338.2010.501793. [DOI] [PubMed] [Google Scholar]
- 15.Fowler CJ, Panicker JN, Drake M, Harris C, Harrison SC, Kirby M, Lucas M, Macleod N, Mangnall J, North A, Porter B, Reid S, Russell N, Watkiss K, Wells M. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:470–477. doi: 10.1136/jnnp.2008.159178. [DOI] [PubMed] [Google Scholar]
- 16.Frohman TC, Davis SL, Beh S, Greenberg BM, Remington G, Frohman EM. Uhthoff’s phenomena in MS-clinical features and pathophysiology. Nat Rev Neurol. 2013;9:535–540. doi: 10.1038/nrneurol.2013.98. [DOI] [PubMed] [Google Scholar]
- 17.Moore KN, Murray S, Malone-Lee J, Wagg A. Rapid urinalysis assays for the diagnosis of urinary tract infection. Br J Nurs. 2001;10:995–1001. doi: 10.12968/bjon.2001.10.15.5264. [DOI] [PubMed] [Google Scholar]
- 18.Rakusa M, Murphy O, McIntyre L, Porter B, Panicker J, Fowler C, Scott G, Chataway J. Testing for urinary tract colonization before high-dose corticosteroid treatment in acute multiple sclerosis relapses: prospective algorithm validation. Eur J Neurol. 2013;20:448–452. doi: 10.1111/j.1468-1331.2012.03806.x. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health Care and Excellence (NICE) Clinical Guideline 148: Urinary incontinence in neurological disease. Management of lower urinary tract dysfunction in neurological disease. 2012. http://guidance.nice.org.uk/cg148.
- 20.Walsh DA, Durance RA. Fatal acute pyelonephritis following pulsed methylprednisolone for rheumatoid arthritis. Ann Rheum Dis. 1990;49:955–956. doi: 10.1136/ard.49.11.955-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupelian AS, Horsley H, Khasriya R, Amussah RT, Badiani R, Courtney AM, Chandhyoke NS, Riaz U, Savlani K, Moledina M, Montes S, O’Connor D, Visavadia R, Kelsey M, Rohn JL, Malone-Lee J. Discrediting microscopic pyuria and leucocyte esterase as diagnostic surrogates for infection in patients with lower urinary tract symptoms: results from a clinical and laboratory evaluation. BJU Int. 2013;112:231–238. doi: 10.1111/j.1464-410X.2012.11694.x. [DOI] [PubMed] [Google Scholar]
- 22.Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, Wilson M, Rohn JL, Malone-Lee J. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013;51:2054–62. doi: 10.1128/JCM.03314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kass EH. Bacteriuria and the diagnosis of infections of the urinary tract; with observations on the use of methionine as a urinary antiseptic. AMA Arch Intern Med. 1957;100:709–14. doi: 10.1001/archinte.1957.00260110025004. [DOI] [PubMed] [Google Scholar]
- 24.Smith GW, Brumfitt W, Hamilton-Miller J. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1983;309:1393–1394. doi: 10.1056/nejm198312013092224. [DOI] [PubMed] [Google Scholar]
- 25.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–468. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 26.Craner MJ, Friese MA, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid sensing ion channel 1 contributes to axonal degeneration in autoimmune CNS inflammation and provides a novel target for neuroprotection in multiple sclerosis. J Neurol. 2008;79:339. [Google Scholar]
- 27.Hutchinson M. There is no such thing as a mild MS relapse. The mild relapse is an Anglo-Saxon delusion - Commentary. Mult Scler. 2012;18:930–931. doi: 10.1177/1352458512450091. [DOI] [PubMed] [Google Scholar]


