Abstract
Maternal food restriction (MFR) during pregnancy affects pulmonary surfactant production in the intrauterine growth-restricted (IUGR) offspring through unknown mechanisms. Since pulmonary surfactant production is regulated by maternal and fetal corticosteroid levels, both known to be increased in IUGR pregnancies, we hypothesized that metyrapone (MTP), a glucocorticoid synthesis inhibitor, would block the effects of MFR on surfactant production in the offspring. Three groups of pregnant rat dams were used (1) control dams fed ad libitum; (2) MFR (50% reduction in calories) from days 10 to 22 of gestation; and (3) MFR + MTP in drinking water (0.5 mg/mL), days 11 to 22 of gestation. At 5 months, the MFR offspring weighed significantly more, had reduced alveolar number, increased septal thickness, and decreased surfactant protein and phospholipid synthesis. These MFR-induced effects were normalized by the antiglucocorticoid MTP, suggesting that the stress of MFR causes hypercorticoidism, altering lung structure and function in adulthood.
Keywords: corticosterone, intrauterine growth restriction, lung development, maternal food restriction, metyrapone
Introduction
“Fetal programming” and the consequent “fetal origins of adult diseases” have been studied extensively in many species.1,2 However, there is only limited information regarding the pulmonary outcomes in offspring following intrauterine growth restriction (IUGR). In general, there is a strong correlation between early growth rates and later altered lung function and pulmonary morbidity.3,4 These pulmonary effects have been grossly attributed to either hypoxia or nutrient restriction during early life. However, the underlying cell-molecular mechanisms that cause these pathophysiologic changes remain unknown.
More recently, it has become apparent that perinatal nutrient restriction constitutively upregulates the fetal hypothalamic–pituitary–adrenal axis,5 disrupting the normal mechanisms that determine fetal development and homeostasis,6 resulting in adult diseases such as obesity, diabetes, hypertension, and chronic lung disease. We speculated that such a chronic hypercorticoid state would specifically disrupt normal lung development, given the physiologic importance of glucocorticoids in this process, consistent with data for the effects of IUGR,7 fetal stress,8 and the effects of exogenous glucocorticoid treatment in the developing lung.9
In our previously published work, we have shown that maternal food restriction (MFR) specifically disrupts the paracrine cross-talk between the lung epithelium and the mesenchyme mediated by parathyroid hormone-related protein (PTHrP) and peroxisome proliferator-activated receptor γ (PPARγ), respectively, that is necessary for normal lung development,10–12 particularly the synthesis of lipids for surfactant production. Alveolar epithelially derived PTHrP acts on its receptor on the mesenchyme, stimulating PPARγ signaling in the adjoining lipofibroblasts, which in turn via paracrine signaling acts on alveolar type II cells to stimulate surfactant synthesis (phospholipid and protein).12 Using the same animal model of MFR, we hypothesized that the resultant excess glucocorticoid activity would affect the offspring adult lung, and that this effect could be blocked by preventing the MFR-induced hypercorticoid state. Therefore, in this study, in addition to determining the effect of MFR on lung morphometry, its effect on surfactant synthesis was determined.
Materials and Methods
Rat Model of MFR
All studies were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and are in accordance with the American Association for the Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. First-time pregnant Sprague Dawley rat dams (Charles River Laboratories, Inc, Hollister, California) were housed in a facility with constant temperature and humidity and on a controlled 12-hour light–12-hour dark cycle. We utilized a previously described model of rat dam 50% food restriction during pregnancy with slight modifications.10,11,13 Briefly, at 10 days of gestation, dams were provided either an ad libitum diet of standard laboratory chow (LabDiet 5001;PMI, Brentwood, Missouri: protein 23%, fat 4.5%, and metabolizable energy = 3030 kcal/kg; control group) or a 50% food-restricted diet (MFR group), as determined by the quantification of the normal intake in the ad libitum fed rats with or without exposure to the endogenous glucocorticoid synthesis inhibitor metyrapone (MTP; Sigma-Aldrich, St Louis, Missouri) in the drinking water (0.5 mg/mL) from day 11 of gestation to delivery (∼day 22). This dosing regimen of MTP has been used effectively in many previous studies.14,15 At day 1 after birth, the litters were separated by gender, and the body weights of individual pups were recorded. To avoid any undue bias toward selecting heavier or lighter pups, the median body weight per litter per gender was determined, and the 4 females closest to the median body weight for the litter were selected for the study; we chose to focus on only 1 gender (females), since there is a well-known gender effect on lung development.16,17 Following delivery, all dams were allowed ad libitum feeds, and the pups were allowed to breast feed ad libitum. In order to eliminate the artifactual effect of MFR on lactation, offspring from both food restricted and control dams were cross-fostered to rat dams fed ad libitum during pregnancy. At postnatal day 21, all offspring were weaned to an ad libitum diet and housed individually. Animals were sacrificed, and lungs collected for further processing at 5 months of age (5M). It is important to point out that water intake was similar in the 3 groups throughout the study period.
Tissue Preparation
Rat lungs were inflated in situ with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at a standard inflation pressure of 20 cm of H2O. The trachea was ligated, and the lungs were removed and placed in 4% PFA for approximately 4 hours at 4°C. The lungs were subsequently transferred to PBS containing 30% sucrose (w/v) until equilibrated (4°C). The lung tissue was either paraffin embedded or frozen in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, California). Frozen sections were cut at 8 μm thickness, and paraffin-embedded sections were cut at 5 μm thickness. Hematoxylin and eosin staining was used to determine the lung morphology.
Lung Morphology
An investigator unaware of the treatment group for each animal sample performed lung morphometry, which was objectively assessed by determining the alveolar count, mean linear intercept, and alveolar septal thickness. For alveolar count determination, 50 randomly selected nonoverlapping fields from sections obtained from similar regions of each lung from each treatment group were examined (12 blocks/condition and 2 blocks/animal.) Each field was viewed at 200-fold magnification, scanned with a digital camera, and projected onto a video monitor. For each field, the number of alveoli was counted visually and expressed per mm2. Mean linear intercept was determined as previously described using the intersection counting method.18 Septal thickness was analyzed with AxioVision image analysis software (Carl Zeiss Microimaging Inc, Thornwood, New York). In brief, slides were examined at 100-fold magnification, and the septal thicknesses of at least 50 alveoli for each section were measured by drawing a straight line crossing the whole width of the septum perpendicular to its course. At least 2 sections from each pup were used, and the average septal thickness was then calculated for each treatment group.
Choline Incorporation into Disaturated Phosphatidylcholine Assay
[Methyl-3H]-choline chloride (NEN Dupont) incorporation into disaturated phosphatidylcholine (DSPC), which is the major surface-active lipid component of surfactant responsible for maintaining the stability of the alveoli, was determined in cultured explants as described previously.19 Briefly, freshly isolated lung explant cultures in Waymouth’s medium in 6-well plates were incubated with 1 μCi/mL of [methyl-3H]-choline chloride for 4 hours. After incubation, explants were washed 3 times with ice-cold PBS. The explants were thoroughly homogenized, and the lung lipids were extracted with chloroform and methanol (2:1, vol/vol). The organic phase was dried under a stream of nitrogen at 60°C, resuspended in 0.5 mL of carbon tetrachloride containing 3.5 mg of osmium tetroxide, and left at room temperature for 15 minutes. The reaction mixture was redried under nitrogen and resuspended in 70 μL of chloroform/methanol (9:1, vol/vol). The lipid extracts were transferred to Silica Gel plates (Kodak, Rochester, New York) and developed in a chloroform–methanol–water (65:25:4, vol/vol) solvent system. Pure dipalmitoyl phosphatidylcholine was used as the chromatographic standard. The developed plates were stained with bromothymol blue, blotted, and vacuum dried for 5 minutes at 90°C. Chromatogram spots corresponding to the migration of saturated phosphatidylcholine were scraped from the plates, and their radioactive contents were determined by liquid scintillation spectrometry. The amounts of [methyl-3H]-choline chloride incorporated into saturated phosphatidylcholine were expressed as disintegrations per minute (dpm) per mg protein per 6 hours.
Triglyceride Uptake Assay
Triolein uptake, a key marker of alveolar lipofibroblast function, was used to quantitate triglyceride uptake by fetal rat lung explants using previously described method.19 Briefly, culture medium was replaced with Dulbecco Modified Eagle Medium containing 20% adult rat serum mixed with [3H]triolein (5 μCi/mL). The explants were incubated at 37°C in 5% CO2–air balance for 4 hours. At the termination of the incubation, the medium was decanted, the explants were rinsed twice with 1 mL of ice-cold PBS, and the tissue was thoroughly homogenized. An aliquot of the tissue homogenate was taken for protein assay, and the remaining tissue homogenate was extracted to determine its neutral lipid content.
Plasma Corticosterone Assay
Plasma corticosterone was determined by radioimmunoassay using a commercial kit (Diagnostic Products Corp, Los Angeles, California). Sensitivity at 90% intercept was 8 ng/mL. Intra- and interassay coefficients of variation were 4% and 6%, respectively.
Protein Expression by Western Blotting
Western blotting was performed according to the previously described methods.19 The specific primary antibodies used included PPARγ (1:2,000; Alexis Biochemicals, San Diego, Calfiornia), surfactant protein B (SP-B) and SP-C (1:2,000 each; Chemicon, Temecula, Calfiornia), cholinephosphate cytidylyltransferase α (CCT-α, 1:2,000; a kind gift from Dr Mallampalli, University of Iowa, Iowa), 11-β-hydroxysteroid dehydrogenase (11β-HSD) 1 and -HSD2 (1:200 each, Santa Cruz, Calfiornia), and glucocorticoid receptor (1:500, Santa Cruz).
Real Time Reverse-Transcriptase Polymerase Chain Reaction
In brief, samples were collected, treated with RNA Later preservative (Ambion/Applied Biosystems, Foster City, California), and total RNA was isolated using the RNAqueous-4PCR kit (Ambion). RNA was DNase-treated and quantitated by light absorbance using a nanodrop spectrophotometer (Nanodrop Instruments, Wilmington, Delaware). Integrity of RNA was assessed from the visual appearance of the ethidium bromide-stained ribosomal bands following fractionation on a 1.2% (wt/vol) agarose-formaldehyde gel and quantitated by light absorbance at 260 nm. Total RNA of 1 μg was reverse transcribed into single-stranded complementary DNA (cDNA) using the TaqMan Gold reverse-transcriptase polymerase chain reation (RT-PCR) kit at 50°C for 30 minutes in a total volume of 20 μL. The PCR reaction mix consisted of 1 μL of 10-fold diluted cDNA, PCR Gold DNA polymerase reagent mix, and optimized for forward and reverse gene-specific primers (900 nmol/L each) with a gene-specific probe (250 nmol/L, FAM dye label). All primer and probe sets were purchased predesigned or, if needed, were custom designed TaqMan Gene Expression Assays (Applied Biosystems). Real-time PCR reactions were run in triplicate on 384-well plates using an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems). Reactions proceeded by activation of DNA polymerase at 95°C for 10 minutes, followed by 38 PCR denaturing cycles at 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute. Data were normalized to 18S ribosomal RNA using the RNA TaqMan Gene Expression Assay. Data were analyzed to select a threshold level of fluorescence that was in the linear phase for PCR product accumulation (the threshold cycle [CT] for that reaction). The CT value for 18S was subtracted from the CT value for the gene of interest to obtain a delta CT (ΔCT) value. The relative fold change for each gene was calculated using the ΔΔCT method.20 Results were expressed as the mean ± standard error and considered significant at P < .05. The RT-PCR probes used included-Rat PPARγ: F-5′-CCAAGTGACTCTGCTCAAGTATGG-3′ and R-5′ CATGAATCCTTGTCCCTCTGATATG-3′ (106 bp); aP2: F-5′-TGAAATCACCCCAGATGACA-3′ and R-5′-TCACGCCTTTCATGACACAT-3′ (155 bp); rat CCT-α: F-5′-CTTTTCATCCCTGTTCCCTCTCT-3′ and R-5′ CCTTAGGTTTAGTGTTGGGATCACA-3′ (92 bp); sterol regulatory element-binding protein 1c (SREBP1c): F-5′-GTGGTCTTCCAGAGGCTGAG-3′ and F-5′-GGGTGAGAGCCTTGAGACAG-3′ (178 bp); rat CCT-α: F-5′-CTTTTCATCCCTGTTCCCTCTCT-3′ and 3′-CATGGCCTTGTCAATGGAAC; and rat 18s: 5′ TTAAGCCATGCATGTCTAAGTAC and 3′-TGTTATTTTTCGTCACTACCTCC.
Statistics
Experimental data were analyzed using 2-way analysis of variance, and as indicated, followed by Tukey post hoc test. A P value of <.05 was considered to indicate statistically significant differences between the experimental and the control groups. Results are expressed as mean ± standard error of the mean (SEM). A minimum of 4 animals/group, taken from 4 separate litters, was used for data analysis.
Results
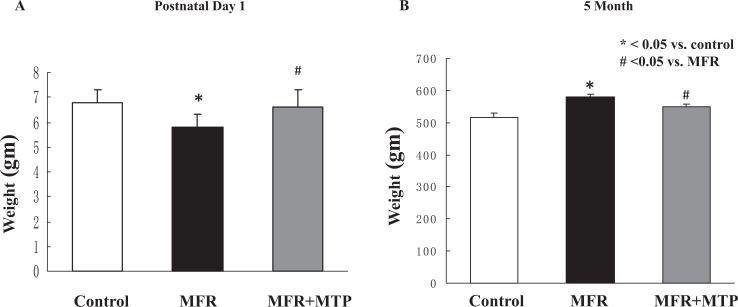
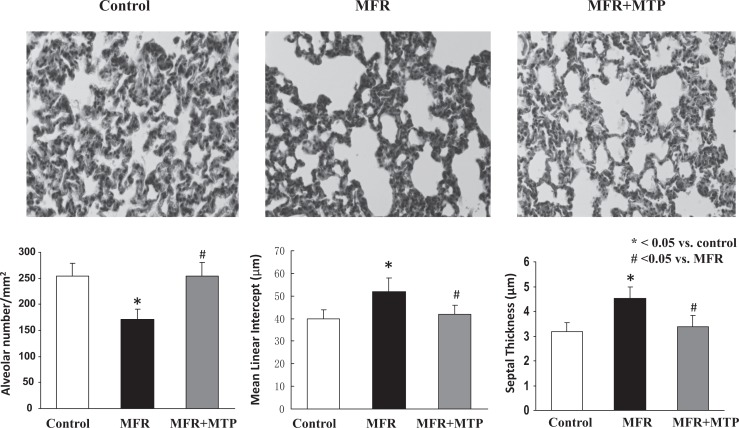
In line with our previously reported data using this model (10, 11), at postnatal day 1, MFR pups weighed significantly less than control pups (5.8 ± 0.5 g, MFR, vs 6.8 ± 0.5 g, control); P < .05), but this difference was normalized with MTP supplementation (6.6 ± 0.7 g; P > .05 vs control group, Figure 1A). Offspring body weights were significantly increased in MFR offspring at 5 months of age, an effect that was partially normalized by the administration of MTP (516 ± 15.2 g vs 580 ± 9.5 g vs 550 ± 9.0, control vs MFR vs MFR + MTP, mean ± SEM, P < .05, MFR vs control, but P > .05, MTP vs control; Figure 1B). At 5 months of age, lung structure was significantly affected by MFR, as indicated by a 32% ± 12% decrease in alveolar number and 30% ± 6% increase in mean linear intercept, accompanied by a 38 ± 14% increase in septal thickness (P < .05, MFR vs control for both), which were normalized by MTP treatment (P > .05, MTP vs control; Figure 2).
Figure 1.
Effect of maternal food restriction (MFR) and metyrapone (MTP) treatment on offspring body weight on postnatal day 1 and at 5 months of age. Offspring body weights were significantly decreased at postnatal day 1 (A) but significantly increased at 5 months (B) in the MFR group, an effect that was normalized by the administration of MTP at both the time points. *P < .05 versus control group and # P < .05 versus MFR group; N = 5.
Figure 2.
Effect of maternal food restriction (MFR) and metyrapone (MTP) treatment on lung cytoarchitecture. At 5 months of age, lung structure was significantly affected by MFR, as indicated by a 32% ± 12% decrease in alveolar number and 30% ± 6% increase in mean linear intercept, accompanied by a 38% ± 14% increase in septal thickness, all of which were normalized by MTP treatment. *P < .05 versus control group and # P < .05 versus MFR group; N = 5.
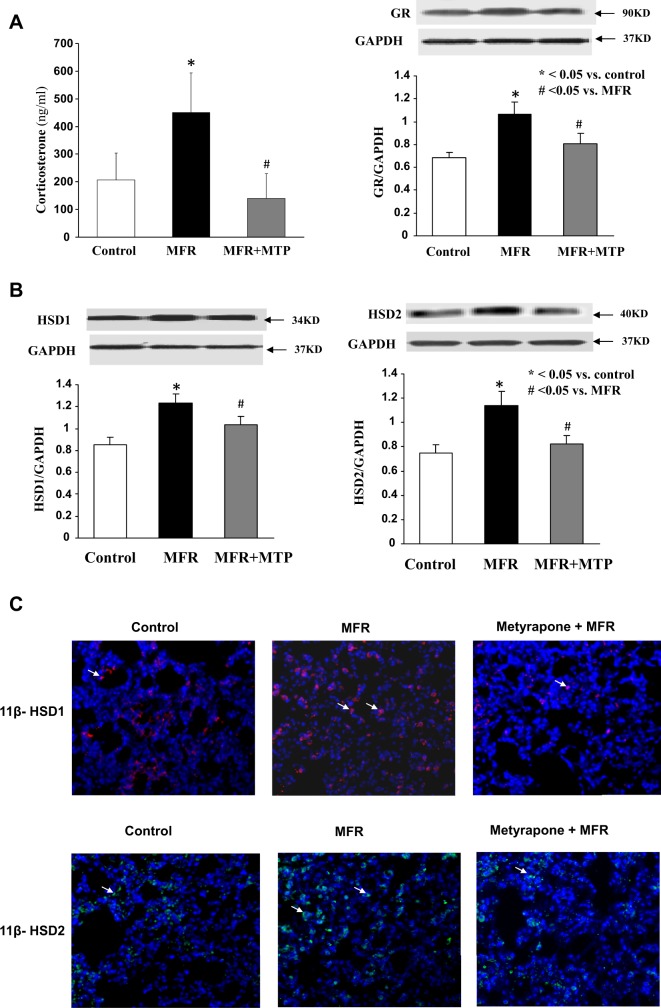
As for the effect of MFR on glucocorticoids and their mechanism of action, it significantly increased the plasma corticosterone level in the e20 dams, an effect that was normalized by MTP (207 ± 97 versus 451 ± 142 versus 140 ± 89 ng/mL, control vs MFR vs MFR + MTP, mean ± SEM, P < .05, Figure 3A). This effect was accompanied by an increase in lung glucocorticoid receptor expression at 5M, which was normalized by MTP treatment (P < .05, Figure 3B). The MFR-stimulated 11β-HSD1 and 2 protein expression at 5M (P < .05, MFR vs control), which was normalized by MTP (P > .05, MTP vs control), confirmed by both Western blotting on protein lysates from the whole lung (Figure 3C) and immunohistochemistry on lung tissue sections stained with anti-11β-HSD1 and 2 antibodies (Figure 3D). Predominant 11β-HSD1 and 2 staining in the corners of the alveoli suggest their predominant localization to alveolar type II cells.
Figure 3.
Effect of maternal food restriction (MFR) and metyrapone (MTP) treatment on maternal corticosterone levels and offspring lung glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase (HSD) 1 and 2 protein levels. Maternal food restriction increased the maternal serum corticosterone level in the e20 dams significantly, an effect that was normalized by MTP (A, N = 8). In the offspring, lung glucocorticoid receptor protein levels increased in offspring at 5 months, which were normalized by MTP treatment (B, N = 4). This was accompanied by increased lung 11β-HSD1 and 2 protein levels, as determined by Western blotting (C) and immunostaining (D, magnification ×20), an effect that was normalized by MTP. N = 4. *P < .05 versus control group and # P < .05 versus MFR group.
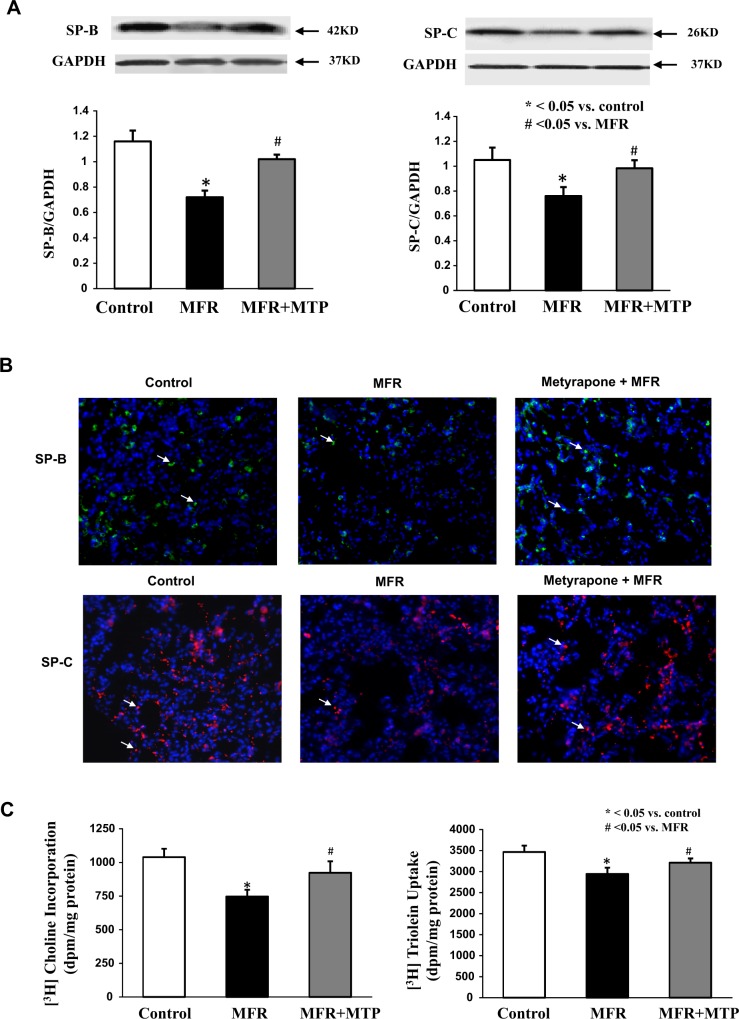
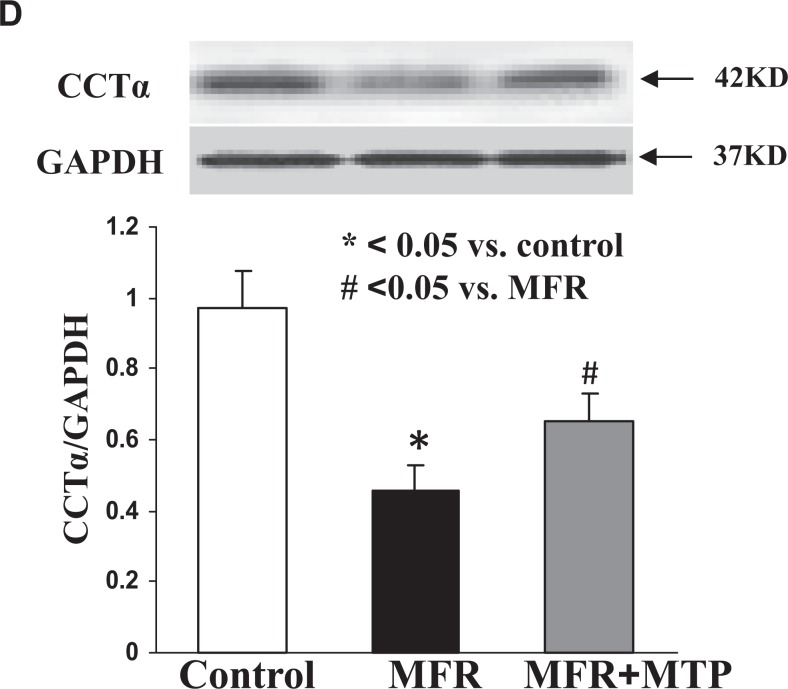
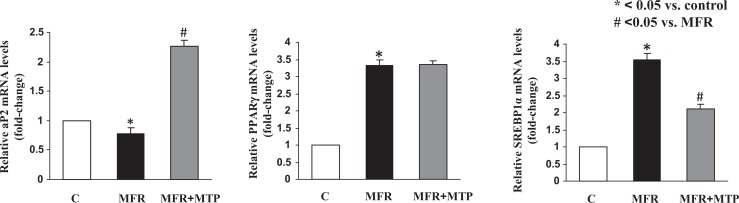
Lung SP-B expression was inhibited 30% ± 6%, and SP-C expression was inhibited 20% ± 9% by MFR (P < .05, MFR vs control for both), which were also normalized by MTP (P > .05, MTP vs control; Figure 4A and B). De novo surfactant phospholipid synthesis based on choline incorporation into DSPC was inhibited by 25% ± 8%, and triolein uptake was inhibited by 15% ± 5% (P < .05, MFR vs control for both), which were normalized by MTP (P > .05, MTP vs control; Figure 4C). Consistent with the effect of MFR on surfactant phospholipid synthesis, it also downregulated CCT-α protein levels (P < .05, Figure 4D), the rate-limiting enzyme in this pathway, an effect that was also normalized by MTP. Turning to the effects of MFR on lung lipid-programming genes, MFR inhibited aP2 at 5M, which was normalized by MTP, but increased the expression of PPARγ and SREBP1c; MTP tended to normalize the MFR effect on SREBP mRNA expression but failed to affect PPARγ expression (P < .05, Figure 5).
Figure 4.
Effect of maternal food restriction (MFR) and metyrapone (MTP) treatment on lung surfactant protein (SP) B and C, and cholinephosphate cytidylyltransferase α (CCT-α) protein levels, choline incorporation into disaturated phosphatidylcholine, and triolein uptake. Lung SP-B level was inhibited by 30% ± 6%, and SP-C by 30% ± 9% by MFR, both of which were normalized by MTP (A; N = 4). These data were corroborated by immunostaining (B, magnification ×20; N = 4). De novo surfactant phospholipid synthesis based on choline incorporation into saturated phosphatidylcholine was inhibited by 25% ± 8%, and triolein uptake was inhibited by 15% ± 5%, both of which were also normalized by MTP (C, N = 5). Consistent with the effect of MFR on surfactant phospholipid synthesis, it also downregulated CCT-α protein levels, the rate-limiting step in this pathway, an effect that was normalized by MTP (D, N = 4). *P < .05 versus control group and # P < .05 versus MFR group.
Figure 4.
(Continued)
Figure 5.
Effect of maternal food restriction (MFR) and metyrapone (MTP) treatment on lung lipid programming genes. Maternal food restriction inhibited aP2 at 5 months of age, which was normalized by MTP, but increased the expression of PPARγ and SREBP; MTP tended to normalize SREBP but did not affect PPARγ. *P < .05 versus control group and # P < .05 versus MFR group; N = 4. PPARγ indicates peroxisome proliferator-activated receptor γ; SREBP, sterol regulatory element-binding protein.
Discussion
Maternal food restriction during pregnancy is physiologically stressful,21–23 causing stimulation of adrenocortical activity, including the production of corticosteroids. In general, this resulted in altered lung structure in the female offspring (males were not examined in this study) at 5M in association with glucocorticoid activation by 11β-HSD1 and 2 and increased glucocorticoid receptor protein. The MFR offspring had significantly decreased alveolar numbers and increased septal thickening. These changes in lung structure were associated with decreased SP levels and phospholipid synthesis. Importantly, all of these lung structural and functional abnormalities were corrected by treatment with the glucocorticoid synthesis inhibitor MTP, indicating that hypercorticoidism played a pivotal role in MFR-induced disruption of lung structure and function.
Food deprivation during fetal development stimulates the hypothalamic–pituitary–adrenal axis, causing constituitive hypercorticoidism in the offspring.5 Among the consequences of elevated corticoids is upregulation of lung 11β-HSD1 expression,24 which promotes glucocorticoid activity, further exacerbating the hypercorticoid state of the animal. In the present study, we observed similar increases in protein expression of both 11β-HSD1 and 2 in type II cells, as indicated by prominent 11β-HSD1 and 2 staining in the corners of the alveoli on immunostaining for these proteins (Figure 3D); however, because the activities of these enzymes are antagonistic, their net contribution to the MFR lung phenotype is indeterminate. Yet, it is clear that the overall glucocorticoid effect was deleterious, given the normalization of alveolar number and septal thickness by MTP.
It has been previously shown that exposure of the developing fetus to excess glucocorticoid in utero and after birth inhibits alveolarization9,25–27 in association with decreased surfactant production.10 In our initial study of the effects of MFR on rat lung development,10 we had also observed decreased alveolar number and septal thickening between birth and 9 months of age in association with decreases in PTHrP and PPARγ expression, which regulates surfactant production. Since we normalized the SP-B and SP-C levels of GAPDH protein levels and surfactant phospholipid synthesis (choline incorporation into DSPC) to protein levels, decreased surfactant synthesis (SP-B and SP-C by both Western blotting and immunostaining and de novo DSPC synthesis by choline incorporation), it suggests that there is not only alveolar reduction but also dysfunctional type II cells in the MFR group and normalization in the MTP-treated group. On one hand, surfactant deficiency per se could have caused alveolar simplification and septal thickening.28 On the other hand, chronic excess glucocorticoid exposure could have inhibited saccularization prenatally and alveolarization postnatally.9,10,25–27 The interactions between these effectors of lung development are complex, and their roles in MFR remain to be further elucidated. From the studies included here, although the mechanisms underlying altered surfactant synthesis in later life following IUGR and exposure to elevated glucocorticoids prenatally can only be speculated, both of these scenarios are known to exert long-term organizational effects during specific “windows” of development,29–31 which can lead to altered surfactant regulation possibly due to general and/or tissue-specific changes in the expression of key genes regulating surfactant expression.
Furthermore, although in this study we did not include an MTP–only treated group, that is, without MFR, parenteral MTP administration (45 mg/kg/dose twice daily) during the last 3 days of rat gestation is known to delay fetal lung maturation and decrease postnatal survival.32 Although in this study we used only female offspring in the study design, MTP is likely to also block MFR-related effects on lung development in males since glucocorticoids are known to affect lung maturation in both males and females, although at different rates,33 but this remains to be tested. However, it is important to point out that in our initial studies, we did not observe any gender-specific effects of either MFR or MTP treatment on offspring lung phenotype, morphologically, and molecularly, when males and females were examined, at postnatal day 21, separately; although this does not rule out gender-specific effects at later time points, the present study was restricted only to females. In this context, although it should be borne in mind that both endogenous17 and exogenous16 glucocorticoids affect the gender difference in the rate of lung development only before birth.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Supported in part by NIH Grants HL75405, HD51857, HD058948, HL55268, HL107118, HD071731, and HD54920.
References
- 1. Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33. [DOI] [PubMed] [Google Scholar]
- 2. Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20(2):132–138. [DOI] [PubMed] [Google Scholar]
- 3. Lipsett J, Tamblyn M, Madigan K, et al. Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatr Pulmonol. 2006;41(12):1138–1145. [DOI] [PubMed] [Google Scholar]
- 4. Maritz GS, Morley CJ, Harding R. Early developmental origins of impaired lung structure and function. Early Hum Dev. 2005;81(9):763–771. [DOI] [PubMed] [Google Scholar]
- 5. Vieau D, Sebaai N, Leonhardt M, et al. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32(suppl 1):S16–S20. [DOI] [PubMed] [Google Scholar]
- 6. Symonds ME, Sebert SP, Budge H. The impact of diet during early life and its contribution to later disease: critical checkpoints in development and their long-term consequences for metabolic health. Proc Nutr Soc. 2009;68(4):416–421. [DOI] [PubMed] [Google Scholar]
- 7. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35(7):730–743. [DOI] [PubMed] [Google Scholar]
- 8. Field T, Diego M. Cortisol: the culprit prenatal stress variable. Int J Neurosci. 2008;118(8):1181–1205. [DOI] [PubMed] [Google Scholar]
- 9. Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD. Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. Am J Respir Crit Care Med. 2001;163(6):1437–1443. [DOI] [PubMed] [Google Scholar]
- 10. Karadag A, Sakurai R, Wang Y, et al. Effect of maternal food restriction on fetal rat lung lipid differentiation program. Pediatr Pulmonol. 2009;44(7):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rehan VK, Sakurai R, Li Y, et al. Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr Pulmonol. 2012;47(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L130–L135. [DOI] [PubMed] [Google Scholar]
- 13. Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R91–R96. [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144(7):3024–3030. [DOI] [PubMed] [Google Scholar]
- 15. Habib S, Gattineni J, Twombley K, Baum M. Evidence that prenatal programming of hypertension by dietary protein deprivation is mediated by fetal glucocorticoid exposure. Am J Hypertens. 2011;24(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol. 1981;141(3):276–287. [PubMed] [Google Scholar]
- 17. Nielsen HC, Torday JS. Sex differences in avian embryo pulmonary surfactant production: evidence for sex chromosome involvement. Endocrinology. 1985;117(1):31–37. [DOI] [PubMed] [Google Scholar]
- 18. Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol. 2010;108(2):412–421. [DOI] [PubMed] [Google Scholar]
- 19. Rehan VK, Wang Y, Sugano S, et al. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L323–L333. [DOI] [PubMed] [Google Scholar]
- 20. Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med (Berl). 2006;84(11):901–910. [DOI] [PubMed] [Google Scholar]
- 21. Budge H, Stephenson T, Symonds ME. Maternal nutrient restriction is not equivalent to maternal biological stress. Curr Drug Targets. 2007;8(8):888–893. [DOI] [PubMed] [Google Scholar]
- 22. Belkacemi L, Jelks A, Chen CH, Ross MG, Desai M. Altered placental development in undernourished rats: role of maternal glucocorticoids. Reprod Biol Endocrinol. 2011;9:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142(5):1692–1702. [DOI] [PubMed] [Google Scholar]
- 24. Dutriez-Casteloot I, Breton C, Coupe B, et al. Tissue-specific programming expression of glucocorticoid receptors and 11 beta-HSDs by maternal perinatal undernutrition in the HPA axis of adult male rats. Horm Metab Res. 2008;40(4):257–261. [DOI] [PubMed] [Google Scholar]
- 25. Tschanz SA, Haenni B, Burri PH. Glucocorticoid induced impairment of lung structure assessed by digital image analysis. Eur J Pediatr. 2002;161(1):26–30. [DOI] [PubMed] [Google Scholar]
- 26. Tschanz SA, Makanya AN, Haenni B, Burri PH. Effects of neonatal high-dose short-term glucocorticoid treatment on the lung: a morphologic and morphometric study in the rat. Pediatr Res. 2003;53(1):72–80. [DOI] [PubMed] [Google Scholar]
- 27. Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate. 1995;68(4):229–245. [DOI] [PubMed] [Google Scholar]
- 28. Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278(16):14291–14298. [DOI] [PubMed] [Google Scholar]
- 29. Singh RR, Cuffe JS, Moritz KM. Short- and long-term effects of exposure to natural and synthetic glucocorticoids during development. Clin Exp Pharmacol Physiol. 2012;39(11):979–989. [DOI] [PubMed] [Google Scholar]
- 30. Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ, Seckl JR. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J. 2012;26(5):1866–74. doi:10.1096/fj.12-203489. [DOI] [PubMed] [Google Scholar]
- 31. Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000;57(4):1412–1417. doi:10.1046/j.1523-1755.2000.00984. [DOI] [PubMed] [Google Scholar]
- 32. Sosenko IR, Lewis PL, Frank L. Metyrapone delays surfactant and antioxidant enzyme maturation in developing rat lung. Pediatr Res. 1986;20(7):672–675. [DOI] [PubMed] [Google Scholar]
- 33. Torday J. Cellular timing of fetal lung development. Semin Perinatol. 1992;16(2):130–139. [PubMed] [Google Scholar]