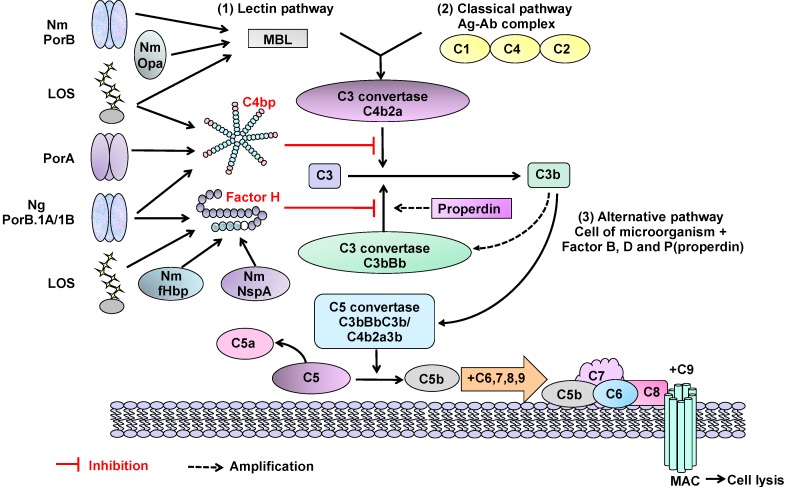
Figure 2.
Interaction of Neisserial surface molecules with the complement system. The interactions of Neisseria molecules with components of the human complement system can have inhibitory or stimulatory effects. (i) Lectin pathway activation: binding of meningococcal (Nm) Opa and PorB proteins by MBL (mannose-binding lectin) is a possible mechanism that accelerates complement activation and increases bacterial killing. The core structure of LOS can also bind MBL, but binding is reduced following LOS sialylation; (ii) Classical pathway down-regulation: Nm PorA, gonococcal (Ng) PorB.1A and PorB.1B and LOS can also bind C4bp (complement regulatory protein C4b-binding protein), which is the main inhibitor of the classical pathway; (iii) Alternative pathway down-regulation: Nm fHbp and NspA, LOS and Ng PorB.1A and PorB.1B bind to complement-inhibitory regulator serum factor H and down-regulate the alternative pathway. Gonococcal strains expressing PorB.1A bind factor H (and/or C4bp) avidly, whereas strains expressing PorB.1B bind factor H weakly. However, sialylation of LOS increases binding of PorB.1B to factor H. The binding of some strains expressing PorB.1B to C4bp can occur regardless of LOS sialylation.