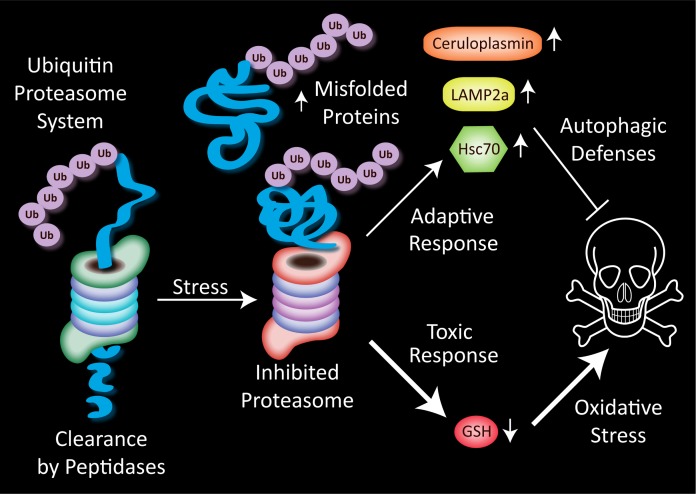
FIGURE 1.
Misfolded proteins are tagged with an ubiquitin tail, linearized, and fed through one end of the barrel-shaped proteasome. Peptides exit the other end of the proteasome and are further degraded by cytoplasmic peptidases into amino acids for recycling into fresh proteins. Stress on the proteasome, such as that induced by proteasome inhibitors, causes the buildup of misfolded proteins that can no longer be degraded. In N2a cells treated with high concentrations of the proteasome inhibitor MG132, two types of responses are elicited in response to the reduced clearance of damaged proteins. A rise in the antioxidant ceruloplasmin and the chaperone-mediated autophagy proteins lysosome-associated membrane protein type-2a (LAMP2a) and heat shock cognate 70 (Hsc70) may serve to defend the cell. Inhibiting autophagic defenses increases the toxicity of both single and dual MG132 hits in this model, suggesting that cells use autophagy as an alternative clearance mechanism when the proteasome is inhibited. Cells also respond to dual hits of severe proteotoxicity with a synergistic loss in glutathione (GSH) defenses. This loss of GSH may increase oxidative toxicity and enhance cell death.