FIGURE 2.
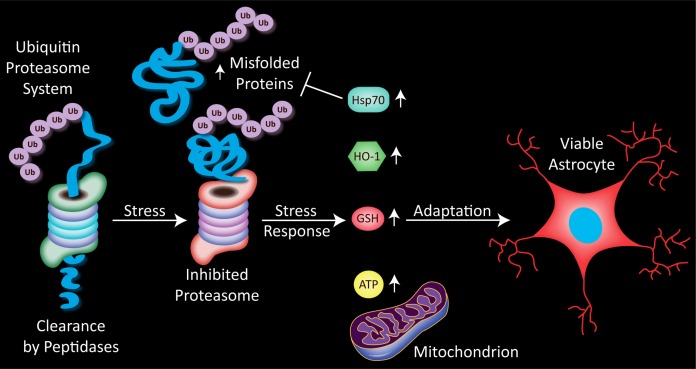
Stress on the proteasome, in the form on inhibition of its normal proteolytic function, increases the cellular burden of damaged proteins. Astrocytes respond to such proteasome inhibition with a rise in the anti-apoptotic heat shock protein 70 (Hsp70). Numerous studies reveal that Hsp70 refolds misfolded proteins or enhances their degradation by the proteasome. A parallel rise in heme oxygenase 1 (HO-1), a generally protective phase 2 enzyme, is also apparent. Astrocytes respond to severe proteotoxicity with glutathione (GSH) loss, unless they have been pre-stressed with MG132, in which case GSH levels are restored. This thiol adaptation serves to increase the number of viable astrocytes and is accompanied by a rise in ATP. Pre-stressed astrocytes are thus both structurally and functionally protected against further proteotoxicity.