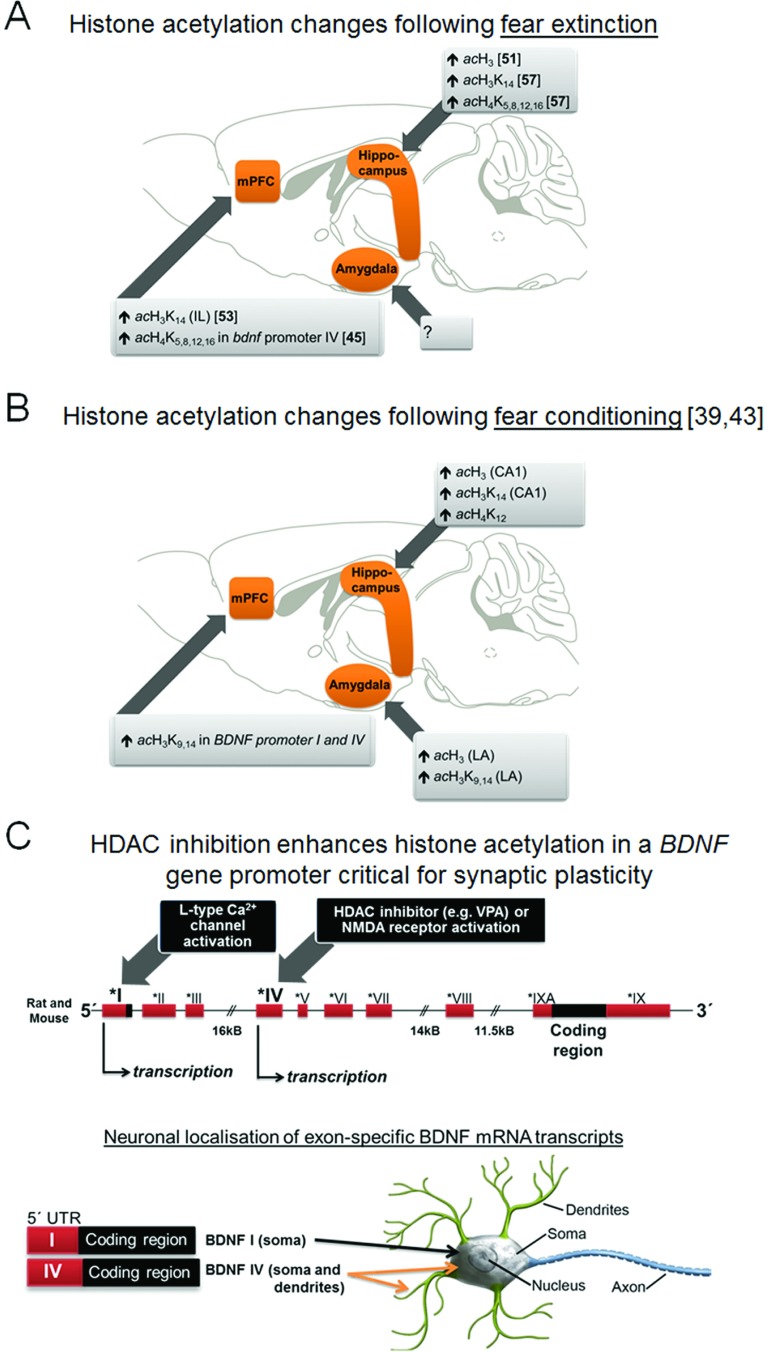
Figure 2. Brain regions displaying enhanced histone acetylation following fear extinction and fear learning.
Published studies have revealed that successful fear extinction (A) and fear conditioning (B) is associated with increases in histone H3 and H4 acetylation in the medial prefrontal cortex (mPFC), hippocampus and amygdala (fear conditioning only; there is no published study concerning fear extinction). Differential epigenetic regulation of BDNF is observed between fear extinction and fear conditioning in the mPFC: extinction-induced increases in histone H4 acetylation are in the promoter region IV of BDNF, whereas fear conditioning increases histone H3 acetylation in promoters of BDNF. This differential histone acetylation in BDNF promoters between fear extinction and fear conditioning may be of significance as acetylated (ac) H3 and H4 are thought to subserve different functions [44]. The rodent Bdnf gene contains at least nine 5′ non-coding exons, each with its own promoter, and a common coding exon (C). Transcription of BDNF transcripts containing exon I or IV has been shown to respond differentially to divergent stimuli; the pan-HDAC inhibitor VPA [82] or NMDA receptor activation predominately increases exon IV-specific mRNA transcripts, whereas L-type voltage-dependent Ca2+ signals seem to mostly increase exon I-specific mRNA transcripts [102]. The neuronal localization of exon I-containing BDNF transcripts is predominately in the soma, whereas that of exon IV-containing BDNF transcripts is in proximal dendrites and in the soma [103]. The targeting of mRNA to specific subcellular compartments, particularly in dendrites is an important feature linked to synaptic plasticity. Thus fear extinction-induced increases in BDNF exon IV transcripts in dendrites [which may be potentiated with HDAC inhibitors (Box 1)] may be a significant mechanism underlying successful fear extinction.