Abstract
Low-dose dopamine inhibits peripheral chemoreceptors and attenuates the hypoxic ventilatory response (HVR) in humans. However, it is unknown: (1) whether it also modulates the haemodynamic reactions to acute hypoxia, (2) whether it also modulates cardiac baroreflex sensitivity (BRS) and (3) if there is any effect of dopamine withdrawal. We performed a double-blind, placebo-controlled study on 11 healthy male volunteers. At sea level over 2 days every subject was administered low-dose dopamine (2 μg kg–1 min–1) or saline infusion, during which we assessed both ventilatory and haemodynamic responses to acute hypoxia. Separately, we evaluated effects of initiation and withdrawal of each infusion and BRS. The initiation of dopamine infusion did not affect minute ventilation (MV) or mean blood pressure (MAP), but increased both heart rate (HR) and cardiac output. Concomitantly, it decreased systemic vascular resistance. Dopamine blunted the ventilatory, MAP and HR reactions (hypertension, tachycardia) to acute hypoxia. Dopamine attenuated cardiac BRS to falling blood pressure. Dopamine withdrawal evoked an increase in MV. The magnitude of the increment in MV due to dopamine withdrawal correlated with the size of the HVR and depended on the duration of dopamine administration. The ventilatory reaction to dopamine withdrawal constitutes a novel index of peripheral chemoreceptor function.
Introduction
Dopamine is an endogenous catecholamine that is often used in clinical practice (Giamouzis et al. 2010; Rafouli-Stergiou et al. 2012), although the clinical benefits of its administration have increasingly been questioned (Friedrich et al. 2005; Chen et al. 2013). Dopamine at low dose exerts its action by stimulating dopamine receptors dispersed widely within the human body (Hussain & Lokhandwala, 2003). Dopamine receptors also play an integral role in the functioning of peripheral chemoreceptors (located in the carotid and aortic bodies; González et al. 1995), which are responsible for the regulation of partial pressures of oxygen and carbon dioxide in the bloodstream (O'Regan & Majcherczyk, 1982). Dopamine has been reported to inhibit afferent signalling from the peripheral chemoreceptors in human patients (van de Borne et al. 1998).
The haemodynamic and ventilatory responses to hypoxia are closely related, which is particularly well seen in patients with heart failure (Niewinski et al. 2013a). As low-dose dopamine attenuates the ventilatory reaction to acute hypoxia (i.e. peripheral chemosensitivity; van de Borne et al. 1998), it could be speculated that it would also affect the reflex haemodynamic responses to hypoxia. Such an analysis might be facilitated by the use of the recently developed methodology for studying peripheral chemoreceptor sensitivity (Niewinski et al. 2013a). If proven correct, it would add to the ongoing discussion on the benefits and perils of the use of dopamine in critical care settings (Rafouli-Stergiou et al. 2012).
Thus far, the effects of dopamine infusion on ventilation have been assessed shortly after its initiation (Welsh et al. 1978; van de Borne et al. 1998). It is unknown whether dopamine withdrawal after prolonged administration would cause any discernible change in the ventilatory or haemodynamic parameters. If a relationship between these parameters and the level of peripheral chemosensitivity could be demonstrated, then reaction to dopamine withdrawal may become a clinically useful and novel index of peripheral chemoreceptor function.
It has been postulated that the response from peripheral chemoreceptors can be evoked not only by acute hypoxia (Chua & Coats, 1995) but might also be tonically aroused during normoxia (Abdala et al. 2012; Sinski et al. 2012; McBryde et al. 2013). This was recently demonstrated in an animal model of heart failure (Del Rio et al. 2013; Schultz et al. 2013), in which carotid body denervation resulted in marked improvement in autonomic indices, including a significant decrease in directly measured sympathetic tone (Marcus et al. 2013). Yet, it is unknown if such tonic peripheral chemoreceptor activation is correlated with the acute response to hypoxia (i.e. reflex chemosensitivity). Low-dose dopamine infusion may facilitate this comparison by inhibition of the tonic activation arising from the peripheral chemoreceptors without interfering with acute hypoxic testing (i.e. assessment of reflex chemosensitivity). Such an evaluation would not be possible using pure oxygen, which is the standard method for assessing the tonic level of peripheral chemoreceptor activation.
As the inverse relationship between peripheral chemosensitivity and cardiac barosensitivity (BRS) has been shown in both animal and human studies (Marshall, 1981; Somers et al. 1991; Ponikowski et al. 1997; Despas et al. 2012), it could be hypothesized that low-dose dopamine-induced inhibition of peripheral chemoreceptors would lead to improvements in BRS. To address the above issues, we performed a double-blind cross-over placebo-controlled study in which we compared ventilatory, haemodynamic and BRS indices in response to low-dose dopamine versus saline infusions in a group of young healthy male volunteers.
Methods
Studied population
After obtaining local Institutional Ethics Committee (Komisja Bioetyczna, Wroclaw Medical University) approval, 11 healthy, non-smoking male volunteers, age 23–40 years (median 26 years), were enrolled into the study. All subjects gave informed consent. The study was performed in accordance with the latest review of the Helsinki Declaration.
Study participants were asked to have a light breakfast and avoid caffeine intake for at least 12 h prior to the examination. During two consecutive days of the study, each subject underwent one infusion per day. All tests followed the same protocol, with the only difference being the intravenous solution used: a low-dose infusion of dopamine (2 μg kg−1 min−1) or an identical volume of normal sterile saline (0.9%) infusion (placebo). The sequence of the administered solutions was randomized and blinded to the researchers (and subjects) performing the testing and calculating the results. A venous catheter was placed into the cephalic vein and primed with saline before connecting to the infusion pump (AP-14; Ascor, Warsaw, Poland).
Measurements
Subjects were examined in the supine position at an ambient temperature of 22°C. A one-way open breathing circuit (Hans Rudolph, Inc., Shawnee, KS, USA) was used during the study. The inhale arm of the circuit served to administer room air or 100% nitrogen gas. Gas switching was controlled silently using a high-pressure electric valve. The exhale arm was connected via a 1000 l min–1 flowhead (MLT3000L; ADInstruments, Sydney, Australia) to a differential pressure transducer (FE141 Spirometer; ADInstruments) for the measurement of breathing rate (BR), tidal volume (TV) and minute ventilation (MV). Heart rate (HR), mean blood pressure (MAP), cardiac output (CO) and systemic vascular resistance (SVR) were continuously and non-invasively recorded using a NexFin device (BMEYE B.V., Amsterdam, Netherlands). A pulse oximeter (Radical-7; Masimo Corp., Irvine, CA, USA) with a lightweight ear clip was used to evaluate blood oxygen saturation ( ). All data were collected at a sampling rate of 1 kHz (16-bit resolution) using PowerLab 16/30 (ADInstruments) and recorded on a laptop computer (Dell Inc., Round Rock, TX, USA).
). All data were collected at a sampling rate of 1 kHz (16-bit resolution) using PowerLab 16/30 (ADInstruments) and recorded on a laptop computer (Dell Inc., Round Rock, TX, USA).
Study protocol
During the entire experiment we measured the following parameters continuously: BR, TV, MV,  , MAP, HR, CO, SVR and the electrocardiogram. The first 5 min of the study protocol was ignored but allowed the subject familiarization with the equipment and environment. The next 10 min of the testing were defined as baseline recording before infusion. Next, an infusion of the previously randomized solution began and the following 15 min was defined as recording during infusion. Subsequently, an assessment of peripheral chemoreceptor sensitivity to intermittent hypoxia was performed using the transient hypoxia method (see below). This phase took approximately 40 min. Ten minutes following the last nitrogen administration (time period defined as baseline prior to infusion withdrawal), the intravenous infusion was stopped and the last 10 min was recorded (defined as recording after infusion withdrawal). The influence of the infusion on the measured parameters was assessed by comparing averaged data from the last 4 min of baseline recording before infusion and from the last 4 min of recording during infusion. For assessment of the effects of the infusion withdrawal we took 4 min immediately preceding and following the infusion termination (Fig. 1A). Additionally, the assessment of the ventilatory reaction to dopamine withdrawal was performed in two subjects using two different durations of dopamine administration (15 and 60 min).
, MAP, HR, CO, SVR and the electrocardiogram. The first 5 min of the study protocol was ignored but allowed the subject familiarization with the equipment and environment. The next 10 min of the testing were defined as baseline recording before infusion. Next, an infusion of the previously randomized solution began and the following 15 min was defined as recording during infusion. Subsequently, an assessment of peripheral chemoreceptor sensitivity to intermittent hypoxia was performed using the transient hypoxia method (see below). This phase took approximately 40 min. Ten minutes following the last nitrogen administration (time period defined as baseline prior to infusion withdrawal), the intravenous infusion was stopped and the last 10 min was recorded (defined as recording after infusion withdrawal). The influence of the infusion on the measured parameters was assessed by comparing averaged data from the last 4 min of baseline recording before infusion and from the last 4 min of recording during infusion. For assessment of the effects of the infusion withdrawal we took 4 min immediately preceding and following the infusion termination (Fig. 1A). Additionally, the assessment of the ventilatory reaction to dopamine withdrawal was performed in two subjects using two different durations of dopamine administration (15 and 60 min).
Figure 1.
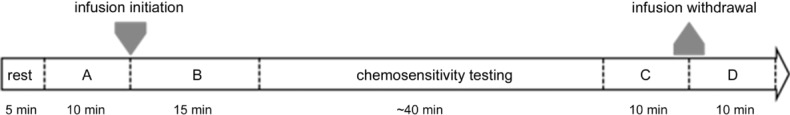
A, baseline recording before infusion; B, recording during infusion; C, baseline prior to infusion withdrawal; D, recording after infusion withdrawal.
Assessment of peripheral chemosensitivity
Peripheral chemoreceptor sensitivity to intermittent hypoxia (hypoxic ventilatory response, HVR) was estimated using the transient hypoxia method (Chua & Coats, 1995). Briefly, subjects were silently switched to breathing with pure nitrogen gas for 10–45 s. This procedure was repeated 5–8 times per study participant, achieving falls in 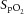 of 99–65%. After each administration of nitrogen subjects were allowed to rest for 5 min breathing room air to allow for the measured parameters to return to baseline levels. The lengths of nitrogen administration were randomized. MV was calculated from breathing rate and tidal volume. Each ventilatory response was calculated as an average from the three largest consecutive breaths following the end of nitrogen administration. HVR was used as a measure of peripheral chemosensitivity and expressed as the slope of the linear regression describing the relationship between the single ventilatory responses and the associated nadirs of
of 99–65%. After each administration of nitrogen subjects were allowed to rest for 5 min breathing room air to allow for the measured parameters to return to baseline levels. The lengths of nitrogen administration were randomized. MV was calculated from breathing rate and tidal volume. Each ventilatory response was calculated as an average from the three largest consecutive breaths following the end of nitrogen administration. HVR was used as a measure of peripheral chemosensitivity and expressed as the slope of the linear regression describing the relationship between the single ventilatory responses and the associated nadirs of 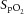 , including the baseline values of MV and
, including the baseline values of MV and 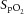 . The baseline values of MV and
. The baseline values of MV and 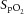 were averaged from the 90 s period prior to each gas administration.
were averaged from the 90 s period prior to each gas administration.
Assessment of haemodynamic response
The haemodynamic response to hypoxia was assessed over the same time course as HVR. The peak HR and peak MAP responses following nitrogen gas administrations were plotted against the corresponding 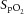 nadirs. The slopes of regression functions describing these relationships were defined as HR slope and MAP slope and reflected the magnitude of the haemodynamic responses to acute hypoxia (Niewinski et al. 2013a).
nadirs. The slopes of regression functions describing these relationships were defined as HR slope and MAP slope and reflected the magnitude of the haemodynamic responses to acute hypoxia (Niewinski et al. 2013a).
Assessment of baroreflex sensitivity
Cardiac BRS was assessed using the sequence method (Parati et al. 1988). Systolic blood pressure (SBP) and R wave to R wave (RR) intervals from 5 min preceding the initiation of the infusion and first nitrogen gas administration (separately for the dopamine and placebo solutions) were used to calculate BRS separately to rising and falling SBP. BRS assessment was based on the analysis of brief (usually lasting for 3–5 cardiac cycles), spontaneous fluctuations in SBP (rises and falls) and corresponding changes in RR intervals. To improve the robustness of the assessment, we decided to report BRS results only for the recordings with more than five sequences, which was possible in 8 out of 11 subjects.
Assessment of heart rate variability
RR intervals taken from 5 min preceding the initiation of the infusion and first nitrogen gas administration (separately for the dopamine and placebo solutions) were analysed with the spectral method (Malliani et al. 1991; using fast Fourier transformation and Hanning windowing) for calculation of the power within the low frequency band (LF, 0.04–0.15 Hz), high frequency band (HF, 0.15–0.40 Hz) and LF/HF ratio. To assess the relative predominance of these components of heart rate variability (HRV), normalized units (n.u.) were used. All premature beats seen during the analysis were corrected by interpolation with previous and following beats.
Data and statistical analysis
Statistica 10 (StatSoft Inc., Tulsa, OK, USA), LabChart Pro (ADInstruments), Nevrocard (Nevrokard Kiauta d.o.o., Izola, Slovenia) and MATLAB (MathWorks, Natick, MA, USA) were used to analyse the data. The statistical comparisons were evaluated using the Wilcoxon matched pairs test. Correlations were calculated using Spearman rank method. Variables are shown as mean and standard error of the mean (SEM), or as median and interquartile range (IQR) where appropriate. A P value < 0.05 is considered statistically significant.
Results
Baseline characteristics in dopamine and placebo arm
The comparison between baseline recording before infusion values of BR, TV, MV, MAP, 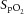 , HR, CO and SVR in the placebo and dopamine arms revealed no significant differences (for more details see Table1). This indicates no changes in the conditions between the two infusions.
, HR, CO and SVR in the placebo and dopamine arms revealed no significant differences (for more details see Table1). This indicates no changes in the conditions between the two infusions.
Table 1.
Median (IQR) values of measured parameters during baseline recording before saline and dopamine infusions
| Baseline before saline infusion | Baseline before dopamine infusion | |
|---|---|---|
| Breathing rate (breaths min–1) | 14.3 (10.8–16.5) | 14.5 (11.4–15.1) |
| Minute ventilation (l min–1) | 10.2 (8.8–11.4) | 9.9 (7.9–11.5) |
| Mean arterial pressure (mmHg) | 91.7 (85.7–97.8) | 95.3 (90.5–102.6) |
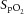 (%) (%) |
95.8 (95.4–97.6) | 96.4 (95.6–97.5) |
| Heart rate (b.p.m.) | 66.1 (56.3–70.6) | 66.8 (52.6–73.2) |
| Cardiac output (l min–1) | 7.2 (6.3–7.5) | 7.7 (6.1–8.5) |
| Systemic vascular resistance (dyn s cm–5) | 1095.2 (919.4–1197.1) | 1052.1 (944.6–1095.7) |
Effects of infusion initiation/termination
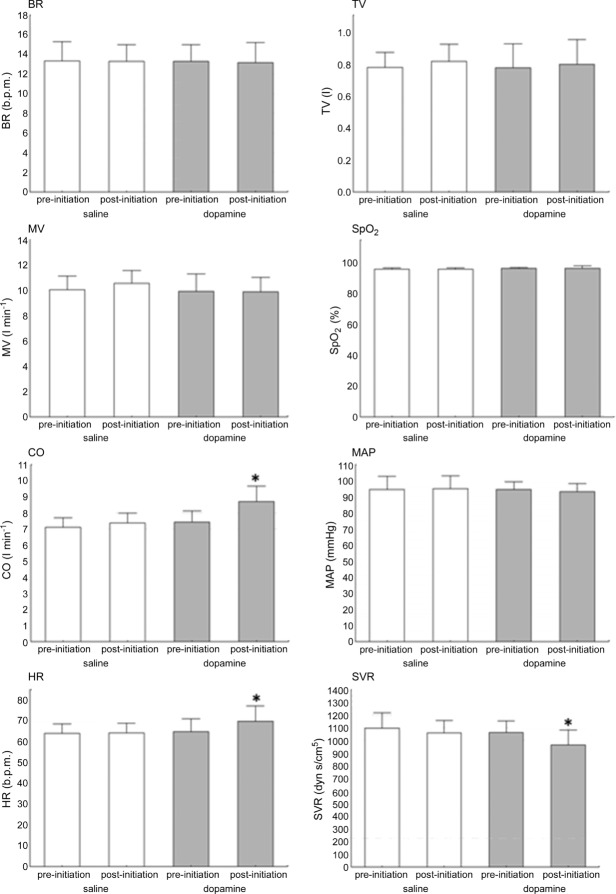
Administration of low-dose dopamine did not influence BR, TV, MV or MAP, but increased both HR (66.8 (52.6–73.2) vs. 71.11 (54.0–82.6) b.p.m., P = 0.008) and CO (7.7 (6.1–8.5) vs. 8.5 (7.8–10.3) l min–1; P = 0.003) and decreased SVR (1052.1 (944.6–1095.7) vs. 923.3 (857.1–1006.8) dyn s cm–5 P = 0.007). By contrast, no significant changes were observed after initiation of the saline infusion (Fig. 2). There was no correlation between changes in the measured parameters (before and during dopamine infusion) and HVR measured during placebo infusion (n.s. for all).
Figure 2.
Open columns, saline infusion initiation; shaded columns, dopamine infusion initiation. *P < 0.05.
The termination of low-dose dopamine caused a significant transient increase in MV (9.1 (8.6–12.2) vs. 11.7 (9.6–12.9) l min−1; P = 0.006). The rise in MV was most prominent after 131 s (118–139) following withdrawal of the infusion. The magnitude of this augmentation of MV post-dopamine infusion correlated with HVR measured during saline infusion (i.e. level of peripheral chemosensitivity) (r = 0.61, P < 0.05). Increased MV resulted from an augmentation of TV (0.75 l (0.56– 0.88) vs. 0.81 l (0.63–1.06), P = 0.003) with no significant change in BR (13.7 (11.3–15.4) vs. 12.8 (12–15.4) breaths min–1, P = 0,18). We also did not observe significant changes in the haemodynamic parameters recorded (HR, MAP, CO, SVR) following dopamine withdrawal (n.s. for all). Detailed haemodynamic data for dopamine and placebo withdrawal are provided in online supplementary material. In contrast to dopamine infusion, the discontinuation of the placebo infusion did not cause significant changes in any of the measured parameters (n.s. for all; Fig. 3).
Figure 3.
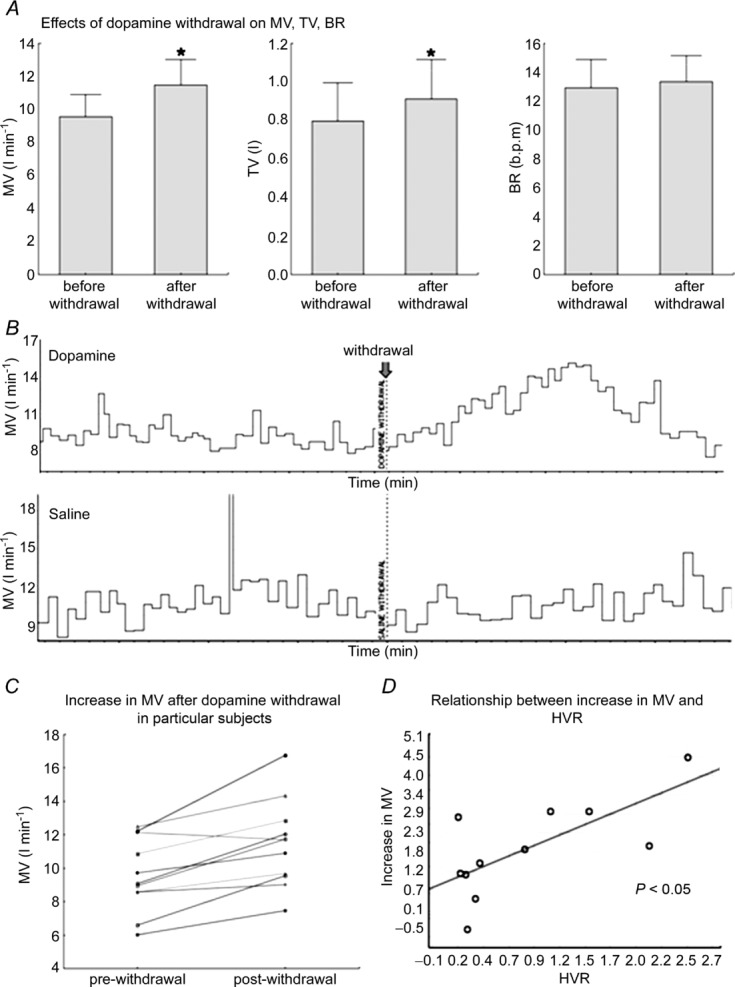
A, effects of dopamine infusion withdrawal on ventilatory parameters. B, example showing the effects of dopamine and placebo withdrawal on MV. C, an increase in MV after dopamine withdrawal presented as individual data. D, correlation between an increase in MV after dopamine withdrawal and hypoxic ventilatory response (HVR). *P < 0.05.
Effects of dopamine on peripheral chemosensitivity, baroreflex and heart rate variability
The infusion of low-dose dopamine reduced significantly the chemoreflex-evoked ventilatory (HVR) and MAP (MAP slope) responses to hypoxia (0.40 (0.26–1.49) vs. 0.16 (0.02–0.24) l min−1/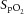 , P = 0.003; 0.49 (0.37–0.8) vs. 0.41 (0.27–0.45) mmHg/
, P = 0.003; 0.49 (0.37–0.8) vs. 0.41 (0.27–0.45) mmHg/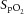 , P = 0.03; saline vs. dopamine, respectively). The infusion of low-dose dopamine also reduced the HR response to hypoxia (HR slope), although this did not quite reach statistical significance (0.56 (0.46–0.64) vs. 0.44 (0.29–0.54) b.p.m./
, P = 0.03; saline vs. dopamine, respectively). The infusion of low-dose dopamine also reduced the HR response to hypoxia (HR slope), although this did not quite reach statistical significance (0.56 (0.46–0.64) vs. 0.44 (0.29–0.54) b.p.m./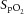 , P = 0.06; Figs 4 and 5).
, P = 0.06; Figs 4 and 5).
Figure 4.
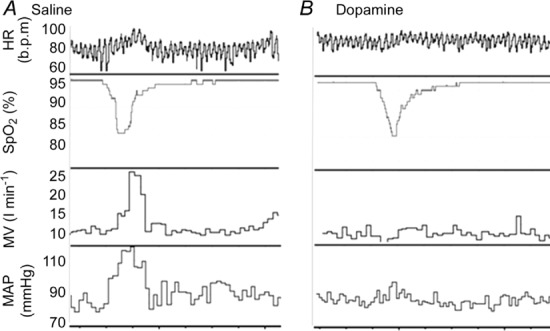
Example of the raw data showing the ventilatory and haemodynamic responses to hypoxia during (A) saline and (B) dopamine infusion.
Figure 5.
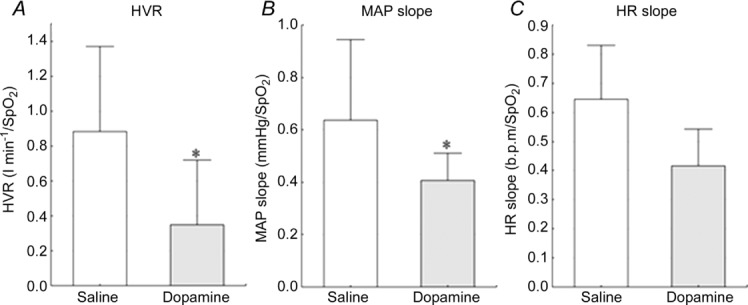
Influence of dopamine on the ventilatory (A) and haemodynamic (B and C) responses to hypoxia. *P < 0.05
Cardiac BRS to falling SBP was significantly decreased after the initiation of the low-dose dopamine infusion compared with placebo (Table2). Interestingly, no change in cardiac BRS was found for rising SBP (Table2). BRS was not calculated in three subjects due to an insufficient number of adequate HR/SBP sequences.
Table 2.
Effects of low-dose dopamine and placebo infusions on cardiac barosensitivity (BRS)
| BRS to rising SBP (ms mmHg–1) |
BRS to falling SBP (ms mmHg–1) |
|||
|---|---|---|---|---|
| Before infusion | On infusion | Before infusion | On infusion | |
| Placebo infusion | 16.07 | 14.65 | 17.5 | 16.43 |
| (12.81–18.38) | (13.83–16.36) | (13.69–19.26) | (13.91–18.11) | |
| Dopamine infusion | 17.33 | 16.5 | 18.54 | 13.42 |
| (14.5–21.11) | (13.52–19.09) | (16.16–19.81) | (12.24–16.34)* | |
Values are given as medians and interquartile ranges. See text for details.
P < 0.05 for before infusion vs. on infusion.
We found no change in LF and HF power of HRV and in LF/HF ratio after the initiation of low-dose dopamine infusion (36 (22–46) vs. 40 (16–54) n.u.; 60 (49–72) vs. 57 (44–80) n.u.; and 1.19 (0.31–0.93) vs. 1.16 (0.20–1.25), respectively, n.s. for all).
Correlations within the indices of peripheral chemosensitivity and barosensitivity
The HVR during the saline infusion correlated with HR slope (r = 0.63, P = 0.04) and showed a trend to correlate with MAP slope (r = 0.55, P = 0.08). We found no correlation between cardiac BRS to rising and falling SBP during both the dopamine (r = 0.57, n.s.) and the placebo infusion (r = 0.19, n.s.).
Relationship between duration of dopamine administration and ventilatory response to dopamine withdrawal
Based on the data obtained from two subjects we found that the magnitude of the ventilatory reaction to the dopamine withdrawal depended on the duration of dopamine administration. The infusions lasting for 60 min resulted in greater ventilatory reactions than the infusions administered for 15 min. This increment in the ventilatory reaction was driven by an increase in TV but not in BR (Table3).
Table 3.
Effect of the duration of dopamine infusion on the ventilatory reaction to dopamine withdrawal
| Change after dopamine withdrawal (%) |
|||
|---|---|---|---|
| Measured parameter | Duration of dopamine infusion (min) | Subject A | Subject B |
| Minute ventilation | 15 | +11 | +10 |
| 60 | +19 | +50 | |
| Tidal volume | 15 | +11 | −1 |
| 60 | +36 | +60 | |
| Breathing rate | 15 | +1 | +19 |
| 60 | −14 | −7 | |
Note that the pronounced increase in minute ventilation observed with long administration is mainly driven by an increment in tidal volume. Subject A has hypoxic ventilatory response of 0.2 l min–1/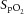 and subject B of 1.1 l min–1/
and subject B of 1.1 l min–1/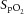 .
.
Discussion
This randomized, double-blind, placebo-controlled study revealed that low-dose dopamine infusion attenuated both the ventilatory reaction and the haemodynamic (HR and MAP) response to acute hypoxia. The second novel finding was an excitatory effect of dopamine withdrawal on MV. The magnitude of this increase in MV correlated with HVR as measured during saline infusion. Finally, we found that the decrease in peripheral chemosensitivity caused by the dopamine administration was accompanied by a decrease in cardiac BRS to falling blood pressure but not to baroreceptor loading.
Ventilatory effects of low-dose dopamine administration
There are two chemosensitive areas in humans – a central site localized at or near the ventral surface of the medulla (Caruana-Montaldo et al. 2000), which is sensitive to hypercapnia, and peripheral regions localized to the carotid (CB) and aortic bodies, which are excited by both hypercapnia and hypoxia (O'Regan & Majcherczyk, 1982). Intravenous dopamine is believed to depress the peripheral chemoreceptors only. This theory was confirmed by Welsh et al. (1978), who revealed that dopamine does not change the hypercapnic ventilatory response. This might be related to the fact that dopamine does not cross the blood–brain barrier (Pardridge, 2005).
The influence of exogenously administered low-dose dopamine on the ventilatory response during hypoxia and the molecular mechanism of action have been described previously (Welsh et al. 1978; Ward & Bellville, 1982; Bascom et al. 1991; González et al. 1995; van de Borne et al. 1998; Iturriaga & Alcayaga, 2004). Dopamine infused at low dose binds mostly to type 2 receptors (D2) on type I glomus cells (GC1) due to much higher affinity as compared with D1 receptors (Lehmann et al. 1983). Activation of D2 receptors leads to a reduction in the release of neurotransmitters from GC1 and therefore to a decrease in the discharge rate of the carotid sinus nerve (CSN) in response to hypoxia (González et al. 1995). In contrast, dopamine infused at a high dose activates both D1 and D2 receptors and results in an increase in CSN discharge rate and MV both in normoxic and in hypoxic conditions (Welsh et al. 1978; González et al. 1995), probably due to activation of the postsynaptic D1 receptors, which overcomes the inhibitory effect of D2 stimulation. We propose that D1 receptors are unlikely to become activated during the low-dose infusion used in our study. This is consistent with seeing a marked decrease in HVR without a significant change in normoxic MV with low-dose dopamine.
Dopamine-related changes in haemodynamic responses to acute hypoxia
The ventilatory response is not the sole consequence of the hypoxic activation of the peripheral chemoreceptors. The acute excitation of the carotid and aortic bodies leads also to an increase in HR and blood pressure (Kumar, 2009). Therefore, blockade of the peripheral chemoreceptors by low-dose dopamine infusion may also blunt the haemodynamic response. Previous studies describe only the respiratory part of the hypoxic response during dopamine administration. In our study, however, we assessed both ventilatory and haemodynamic changes simultaneously with accompanying intermittent decreases of 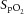 . Using this method we revealed that the reduction in HVR caused by the dopamine infusion is accompanied by reductions in the MAP and HR responses to hypoxia (hypertension, tachycardia), although the HR response did not quite reach statistical significance (P = 0.06). However, this decrease was seen consistently despite an increased HR resulting from the dopamine infusion itself. As HVR showed positive correlations with both the HR and the MAP slopes, we suggest that all these reflex components can be down-modulated by a dopaminergic mechanism at the level of the peripheral chemoreceptor. While we assume that this interruption is caused by dopamine acting at the receptor level, it is difficult to speculate at which stage any divergence of the ventilatory and haemodynamic signals takes place. Some authors suggest that it is within the CB, where different populations of glomus cells might be responsible for ventilatory versus sympathetic control (Paton et al. 2013). On the other hand, the central site of termination of peripheral chemoreceptor afferents within the nucleus tractus solitarii could also be responsible for a differential modulation of respiratory and cardiovascular/sympathetic reactions to hypoxia (Sapru, 1996; Paton, 1998a,b1998b). However, a recent study performed in humans after CB resection suggested that the dissociation between ventilatory, cardiac and blood pressure responses to hypoxia might be related to different function of carotid versus aortic bodies (Niewinski et al. 2013b).
. Using this method we revealed that the reduction in HVR caused by the dopamine infusion is accompanied by reductions in the MAP and HR responses to hypoxia (hypertension, tachycardia), although the HR response did not quite reach statistical significance (P = 0.06). However, this decrease was seen consistently despite an increased HR resulting from the dopamine infusion itself. As HVR showed positive correlations with both the HR and the MAP slopes, we suggest that all these reflex components can be down-modulated by a dopaminergic mechanism at the level of the peripheral chemoreceptor. While we assume that this interruption is caused by dopamine acting at the receptor level, it is difficult to speculate at which stage any divergence of the ventilatory and haemodynamic signals takes place. Some authors suggest that it is within the CB, where different populations of glomus cells might be responsible for ventilatory versus sympathetic control (Paton et al. 2013). On the other hand, the central site of termination of peripheral chemoreceptor afferents within the nucleus tractus solitarii could also be responsible for a differential modulation of respiratory and cardiovascular/sympathetic reactions to hypoxia (Sapru, 1996; Paton, 1998a,b1998b). However, a recent study performed in humans after CB resection suggested that the dissociation between ventilatory, cardiac and blood pressure responses to hypoxia might be related to different function of carotid versus aortic bodies (Niewinski et al. 2013b).
Apart from the sympathetic nervous system one must acknowledge the Hering–Breuer reflex and its influence on the HR and MAP responses to hypoxia. This reflex originating from the pulmonary stretch receptors and stimulated by hyperventilation leads to tachycardia and vasodilatation (Ursino & Magosso, 2000; Kumar, 2009). It is possible that the dopamine infusion influences the haemodynamic response by diminishing the ventilatory response to hypoxia and hence suppresses the role of the Hering–Breuer reflex. While this might assist in explaining the reduction in HR slope, it is not in concordance with the MAP slope decrease. As cardiac BRS to rising blood pressure did not change after initiation of the dopamine infusion, we believe that the influence of the baroreflex on the magnitude of the haemodynamic responses to hypoxia in our study is negligible. A final issue is that the hypoxic stimulus is likely to have a direct inhibitory effect on the cardiac pacemaker cells (Marshall & Metcalfe, 1988) and vascular smooth muscle tone (Umbrello et al. 2013) – this might lead to a generalized underestimation of the haemodynamic responses during both the dopamine and the placebo infusions.
We recently found that CB denervation in human patients results in the dissociation of BP versus HR responses to hypoxia (Niewinski et al. 2013b). It is also well known that the aortic bodies are reactive to dopamine agonists and antagonists (Smatresk & Lahiri, 1982). In this context, the reductions in both MAP and HR slopes seen in our study suggest that dopamine exerts its haemodynamic actions not only through the carotid bodies but also through the aortic bodies. In contrast, the ventilatory response in humans is solely mediated by the carotid bodies (Kumar, 2009).
Comparison with previous studies
The effects of the initiation of low-dose dopamine (2–5 μg kg−1 min−1) on normoxic MV, HR and MAP in healthy humans have been described previously. van de Borne et al. (1998) reported no changes in both MV and MAP but increased HR, while Welsh et al. (1978) described a significant decrease in MV but no changes in MAP and HR. Our observations are concordant with those made by van de Borne, which are consistent with the proposed mode of action of dopamine discussed above. Of interest, none of these studies evaluated the influence of low-dose dopamine on SVR, which is positively affected by high doses of the drug (>10 μg kg−1 min−1; Stetson & Reading, 1977). In our study, low-dose dopamine caused vasodilatation as proven by drop in SVR, which was balanced by a concomitant increase in CO, thus resulting in relatively stable blood pressure. Discrepancies in the cited studies can be most likely attributed to the differences in the dopamine dosing, relatively low numbers of studied subjects and often subtle dopamine-related changes in MV seen in healthy humans.
Effects of dopamine on tonic and acute components of peripheral chemoreflex
Our study provides insight into the issue of sensitivity and tonicity of the peripheral chemoreflex. The dopamine infusion is expected to eliminate any tonic aspect of peripheral chemoreflex drive as with hyperoxia (Sinski et al. 2012). Both methods have side effects, including tachycardia and hypertension for dopamine and hyperoxia, respectively, that may influence findings and interpretations. However, it is only the low-dose dopamine method that enables a concomitant study of the acute hypoxic response, and thus assessment of both ventilatory and haemodynamic components of reflex chemosensitivity. In our study, we did not find a significant relationship between HVR and MV changes after initiation of the dopamine infusion (index of tonic activation). This is consistent with the notion that reflex sensitivity of the peripheral chemoreceptors is not necessarily related to its tonic activation (Paton et al. 2013). On the other hand, we did not notice a significant change in MV after the initiation of dopamine infusion, which could be related to the very low level (or absence) of any tonic activity in healthy individuals (Stickland et al. 2008). A significant decrease in sympathetic tone was seen after CB denervation in the animal model (Marcus et al. 2013). However, the animals studied developed a high level of sympathetic drive and increased chemosensitivity to hypoxia and hypercapnia at baseline due to introduction of heart failure state (Del Rio et al. 2013).
Despite the lack of significant changes in MV after dopamine initiation, we observed a significant drop in SVR. One could hypothesize that this fall is not only due to the direct influence of dopamine on several subtypes of dopamine receptors present in peripheral vasculature (Polakowski et al. 2004; Armando et al. 2011), but can also arise from inhibition of the haemodynamic arm of the peripheral chemoreflex. Therefore, a reduction of SVR could be related to modulation of the sympathetic tone to blood vessels by chemoreceptor deactivation, as shown by Sinski et al. (2012). Admittedly, van de Borne et al. (1998) did not observe a significant decrease in sympathetic nerve activity during normoxic dopamine infusion in healthy individuals, but during hypoxic state noted the lack of expected sympathoactivation. HRV analysis performed in our study showed no significant changes in indices of sympathetic/parasympathetic activity (LF power, HF power, LF/HF ratio) after initiation of dopamine infusion. However, we regard these results as inconclusive because of a possible direct influence of dopamine on sinus node β-receptors (Endoh, 1975). A more complete picture would be expected to be obtained from subjects with presumably high chemosensitivity and tonic activity, e.g. patients with congestive heart failure (Niewinski et al. 2013a) or possibly hypertension (McBryde et al. 2013).
Dopamine withdrawal
While the results of the initiation of low-dose dopamine infusion have been described previously (Welsh et al. 1978; Bascom et al. 1991; van de Borne et al. 1998), the effects of its withdrawal have not been reported so far. In the present study, withdrawal of the prolonged dopamine infusion (lasting nearly 60 min) resulted in a significant temporary rise in MV. The magnitude of this increase correlated with the level of HVR measured during the placebo infusion (i.e. chemosensitivity), suggesting a similar mechanism was involved. While we can only speculate as to the mechanism of this phenomenon, we hypothesize that the withdrawal reaction could be caused by the sudden release of the excitatory neurotransmitters accumulating in GC1 cells. Neurotransmitters of the CB are constantly synthesized in GC1 cells and stored in vesicles in the cytoplasm (Gonzalez et al. 1994). Activation of D2 receptors on GC1 cells causes hyperpolarization as calcium currents are blocked (Carroll et al. 2005). This minimizes the release of CB neurotransmitters (González et al. 1995). It could be expected that the neuroactive substances accumulate in the GC1 cells and when the inhibiting effect of dopamine subsides the release rate of these neurotransmitters rises temporarily, increasing the discharge rate in the CSN and resulting in a hyperventilatory response (Fig. 3). This concept is further supported by the fact that the magnitude of the ventilatory response to dopamine withdrawal depends on the duration of dopamine administration (see Table3). As individuals with sensitized peripheral chemoreceptors are also expected to have a higher rate of neurotransmitter synthesis (Soulier et al. 1997; Wang et al. 1998), the response to the withdrawal of dopamine infusion could be more pronounced in these individuals, which is reflected in our study by the positive correlation between HVR and the magnitude of the MV response to dopamine withdrawal. No such relationship was found for the haemodynamic components of the withdrawal reaction. This could be due to the postulated heterogeneity of the glomus cells (Paton et al. 2013) and/or neurotransmitters responsible for ventilatory and haemodynamic arms of the peripheral chemoreflex. Another possibility for the lack of concomitant ventilatory and haemodynamic responses to dopamine withdrawal could be related to the differential action of dopamine on carotid versus aortic chemoreceptors. However, a recent study has suggested the existence of contrasting patterns of response to hypoxia between carotid and aortic bodies (Niewinski et al. 2013b).
Dopamine and baroreflex sensitivity
The sequential method used in our study (Parati et al. 1988), which is based on the analysis of very brief and spontaneous fluctuations in blood pressure, allowed for BRS assessment despite unchanged averaged MAP observed during dopamine infusion. The inverse relationship between peripheral chemosensitivity and BRS has already been shown in both healthy and congestive heart failure subjects (Somers et al. 1991; Ponikowski et al. 1997). It was also shown that the transient blockade of the peripheral chemoreceptors with hyperoxia temporarily increased BRS (Ponikowski et al. 1997). Therefore, attenuation of peripheral chemoreceptor activity by low-dose dopamine infusion should enhance BRS. In our study, however, we found a decrease in cardiac BRS but only to falling SBP. Since cardiac BRS to rising BP is controlled mostly by the parasympathetic system whereas the cardiac BRS to falling BP involves activation of the cardiac sympathetic system (La Rovere et al. 2008), it could be speculated that dopamine administration modulated predominantly the sympathetic component of the autonomic system. This distinction is further supported by the lack of a correlation between cardiac BRS to rising and falling blood pressure. Thus, the attenuation of cardiac BRS could be related to cardiac sympathoinhibition attributed to: (1) dopamine itself (Mannelli et al. 1997; Kaya et al. 2003) or (2) a dopamine-mediated decrease in chemoreceptor activity (Ciarka et al. 2005; Sinski et al. 2012). On the other hand, the observed decrease in cardiac BRS could simply be an effect of increased HR (Ward et al. 2006) secondary to the dopamine infusion and movement to the non-linear portion of the baroreflex function curve. Our findings differ from those of Somers et al. (1991), possibly because we did not assess sympathetic tone after simultaneous activation of baroreceptors and chemoreceptors, but rather looked at spontaneous cardiac BRS in response to chemoreflex modulation. It is also difficult to compare our data with the results obtained by Ponikowski et al. (1997), as we used different methodology to assess cardiac baroreflex and did so not in heart failure patients but in young, healthy subjects with presumably no impairment of baroreflex function. Thus, the effect of dopamine on cardiac BRS using the sequence method remains inconclusive.
Study limitations
As expected, some systemic actions of low-dose dopamine were observed (increase in HR and cardiac output). These changes might to some extent influence HR responses (tachycardia) to acute hypoxia and hence the measurement of HR slope during dopamine infusion. Isocapnia was not maintained during evaluation of the ventilatory and haemodynamic responses to hypoxia. However, such a poikilocapnic approach has been previously validated and proven to be clinically useful in healthy humans and patients with systolic heart failure (Chua & Coats, 1995; Niewinski et al. 2013a).
Conclusions
We present novel phenomena related to the dopamine-induced inhibition of the peripheral chemoreceptors. First, we found that low-dose dopamine reduces both the ventilatory and the HR/MAP reactions to hypoxia, which could be of particular importance, especially in critically ill patients in whom dopamine is still used. In the event of acute respiratory failure (leading to hypoxia) such patients might be lacking the compensatory effects of tachycardia and hypertension. On the other hand, decreased afterload and HR responses to hypoxia might lead to improvements in stroke volume and diastolic coronary perfusion, respectively, which might benefit some of the dopamine-treated patients. However, as recently shown (Chen et al. 2013) administration of low-dose dopamine had no impact on diuresis, renal function and prognosis in decompensated heart failure patients with renal dysfunction.
Moreover, we found a decrease in cardiac BRS that might be related to the modulation of the sympathetic system. Finally, we describe a positive ventilatory reaction to dopamine withdrawal, which could serve as a novel index of the degree of peripheral chemoreceptor activity between individuals.
Key points
Low-dose dopamine reduces the ventilatory response to acute hypoxia both in animal and in human studies.
In this study we show that low-dose dopamine also attenuates the haemodynamic responses (tachycardia, hypertension) to acute hypoxia in healthy humans.
Moreover, we found that dopamine withdrawal results in a temporary increase in minute ventilation.
The magnitude of the increase in minute ventilation after dopamine withdrawal correlates with the degree of ventilatory response to acute hypoxia and depends on the duration of dopamine administration.
Dopamine may provide a novel method for assessing differences in the level of peripheral chemoreceptor activity, which has important clinical implications given the recently reported pathological role of the carotid body in cardiovascular diseases in animals and humans.
Translational perspective.
The efficacy of low-dose dopamine in the treatment of critically ill patients remains an unresolved issue. Randomized clinical trials performed mostly in acute heart failure patients have provided conflicting and rather discouraging results. While low-dose dopamine exerts some diuretic and minor inotropic properties, these struggle to translate into clinically meaningful benefits. An influence of low-dose dopamine on peripheral chemoreceptors is rarely appreciated among clinicians. In our study we showed that such intervention through the inhibition of chemoreceptive cell function results in diminished ventilatory and haemodynamic responses to acute hypoxia. This could be of importance in severely ill patients in whom compensatory reactions to hypoxia (hyperventilation, tachycardia and hypertension) are necessary for adequate tissue perfusion. Similarly, low-dose dopamine, could complicate the process of weaning from mechanical ventilation by inducing a state of inappropriate sensing of hypoxia.
On the other hand, recent studies have suggested that denervation of the CB results in favourable modulation of autonomic tone in animal models of hypertension and heart failure. Our findings might support this approach by showing a significant decrease in systemic vascular resistance with a corresponding drop in peripheral chemosensitivity. However, a direct vasodilatory effect of low-dose dopamine administration cannot be excluded.
Acknowledgments
None declared.
Glossary
- BR
breathing rate
- BRS
baroreflex sensitivity
- CB
carotid body
- CO
cardiac output
- CSN
carotid sinus nerve
- GC1
type I glomus cell
- HF
high frequency band
- HR
heart rate
- HRV
heart rate variability
- HVR
hypoxic ventilatory response
- IQR
interquartile range
- LF
low frequency band
- MAP
mean arterial pressure
- MV
minute ventilation
- RR interval
R wave to R wave interval
- SEM
standard error of the mean
- SBP
systolic blood pressure
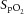
blood oxygen saturation
- SVR
systemic vascular resistance
- TV
tidal volume.
Additional information
Conflict of interest
The authors have no conflict of interest to declare.
Author contributions
The experiments were performed in the Laboratory for Applied Cardiovascular Research at the Department of Cardiology, 4th Military Hospital, Wroclaw, Poland. P.N.: conception and design of the experiments, collection, analysis and interpretation of data, drafting the article. S.T.: conception and design of the experiments, collection, analysis and interpretation of data, drafting the article. W.B.: data interpretation and analysis, revision of the manuscript. J.F.R.P.: data interpretation and analysis, revision of the manuscript. Piotr Ponikowski: conception and design of the experiments, drafting the article, revision of the manuscript. All authors approved the final version of the manuscript. All persons designated as authors qualify for authorship. All those who qualify for authorship are listed.
Funding
None declared.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol. 2011;1:1075–1117. doi: 10.1002/cphy.c100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom DA, Clement ID, Dorrington KL, Robbins PA. Effects of dopamine and domperidone on ventilation during isocapnic hypoxia in humans. Respir Physiol. 1991;85:319–328. doi: 10.1016/0034-5687(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Boyle KM, Wasicko MJ, Sterni LM. Dopamine D2 receptor modulation of carotid body type 1 cell intracellular calcium in developing rats. Am J Physiol Lung Cell Mol Physiol. 2005;288:L910–916. doi: 10.1152/ajplung.00414.2003. [DOI] [PubMed] [Google Scholar]
- Caruana-Montaldo B, Gleeson K, Zwillich CW. The control of breathing in clinical practice. Chest. 2000;117:205–225. doi: 10.1378/chest.117.1.205. [DOI] [PubMed] [Google Scholar]
- Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, Lewinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua TP, Coats AJ. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur J Clin Invest. 1995;25:887–892. doi: 10.1111/j.1365-2362.1995.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Ciarka A, Najem B, Cuylits N, Leeman M, Xhaet O, Narkiewicz K, Antoine M, Degaute JP, van de Borne P. Effects of peripheral chemoreceptors deactivation on sympathetic activity in heart transplant recipients. Hypertension. 2005;45:894–900. doi: 10.1161/01.HYP.0000161875.32767.ac. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despas F, Lambert E, Vaccaro A, Labrunee M, Franchitto N, Lebrin M, Galinier M, Senard JM, Lambert G, Esler M, Pathak A. Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens. 2012;30:753–760. doi: 10.1097/HJH.0b013e328350136c. [DOI] [PubMed] [Google Scholar]
- Endoh M. Effects of dopamine on sinus rate and ventricular contractile force of the dog heart in vitro and in vivo. Br J Pharmacol. 1975;55:475–486. doi: 10.1111/j.1476-5381.1975.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschope C, Triposkiadis F. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- González C, López-López J, Obeso A, Pérez-García MT, Rocher A. Cellular mechanisms of oxygen chemoreception in the carotid body. Respir Physiol. 1995;102:137–147. doi: 10.1016/0034-5687(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev. 2004;47:46–53. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kaya D, Ellidokuz E, Onrat E, Ellidokuz H, Celik A, Kilit C. The effect of dopamine type-2 receptor blockade on autonomic modulation. Clin Auton Res. 2003;13:275–280. doi: 10.1007/s10286-003-0097-3. [DOI] [PubMed] [Google Scholar]
- Kumar P. Systemic effects resulting from carotid body stimulation–invited article. In: Gonzalez C, Nurse C, Peers C, editors. Arterial Chemoreceptors. Dordrecht: Springer; 2009. pp. 223–233. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Briley M, Langer SZ. Characterization of dopamine autoreceptor and [3H]spiperone binding sites in vitro with classical and novel dopamine receptor agonists. Eur J Pharmacol. 1983;88:11–26. doi: 10.1016/0014-2999(83)90387-4. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Lazzeri C, Ianni L, La Villa G, Pupilli C, Bellini F, Serio M, Franchi F. Dopamine and sympathoadrenal activity in man. Clin Exp Hypertens. 1997;19:163–179. doi: 10.3109/10641969709080813. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2013 doi: 10.1113/jphysiol.2013.266221. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Interaction between the responses to stimulation of peripheral chemoreceptors and baroreceptors: the importance of chemoreceptor activation of the defence areas. J Auton Nerv Syst. 1981;3:389–400. doi: 10.1016/0165-1838(81)90077-1. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Engelman Z, Fudim M, Tubek S, Paleczny B, Jankowska E, Banasiak B, Sobotka P, Ponikowski P. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J Card Fail. 2013a;19:408–415. doi: 10.1016/j.cardfail.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC, Paton JF, Ponikowski P. Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol. 2013b doi: 10.1113/expphysiol.2013.075580. doi: 10.1113/expphysiol.2013.075580. [DOI] [PubMed] [Google Scholar]
- O'Regan R, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol. 1982;100:23–40. doi: 10.1242/jeb.100.1.23. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–222. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. Convergence properties of solitary tract neurones driven synaptically by cardiac vagal afferents in the mouse. J Physiol. 1998a;508:237–252. doi: 10.1111/j.1469-7793.1998.237br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fibre stimulation in the mouse. J Neurophysiol. 1998b;79:2365–2373. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz K. Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep. 2013;15:273–280. doi: 10.1007/s11906-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakowski JS, Segreti JA, Cox BF, Hsieh GC, Kolasa T, Moreland RB, Brioni JD. Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats. Clin Exp Pharmacol Physiol. 2004;31:837–841. doi: 10.1111/j.1440-1681.2004.04095.x. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJ. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–2594. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- Rafouli-Stergiou P, Parissis JT, Anastasiou-Nana M. Inotropes for the management of acute heart failure patients with renal dysfunction. Still an option? Expert Opin Pharmacother. 2012;13:2637–2647. doi: 10.1517/14656566.2012.749859. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol. 1996;410:357–364. [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep. 2013;15:356–362. doi: 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A, Gaciong Z. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res. 2012;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- Smatresk NJ, Lahiri S. Aortic body chemoreceptor responses to dopamine, haloperidol, and pargyline. J Appl Physiol. 1982;53:596–602. doi: 10.1152/jappl.1982.53.3.596. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier V, Gestreau C, Borghini N, Dalmaz Y, Cottet-Emard JM, Pequignot JM. Peripheral chemosensitivity and central integration: neuroplasticity of catecholaminergic cells under hypoxia. Comp Biochem Physiol A Physiol. 1997;118:1–7. doi: 10.1016/s0300-9629(96)00369-6. [DOI] [PubMed] [Google Scholar]
- Stetson JB, Reading GP. Avoidance of vascular complications associated with the use of dopamine. Can Anaesth Soc J. 1977;24:727–733. doi: 10.1007/BF03006717. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbrello M, Dyson A, Feelisch M, Singer M. The key role of nitric oxide in hypoxia: hypoxic vasodilation and energy supply-demand matching. Antioxid Redox Signal. 2013;19:1690–1710. doi: 10.1089/ars.2012.4979. [DOI] [PubMed] [Google Scholar]
- Ursino M, Magosso E. Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model. Am J Physiol Heart Circ Physiol. 2000;279:H149–165. doi: 10.1152/ajpheart.2000.279.1.H149. [DOI] [PubMed] [Google Scholar]
- van de Borne P, Oren R, Somers VK. Dopamine depresses minute ventilation in patients with heart failure. Circulation. 1998;98:126–131. doi: 10.1161/01.cir.98.2.126. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Dinger B, Fidone SJ, Stensaas LJ. Changes in tyrosine hydroxylase and substance P immunoreactivity in the cat carotid body following chronic hypoxia and denervation. Neuroscience. 1998;83:1273–1281. doi: 10.1016/s0306-4522(97)00440-5. [DOI] [PubMed] [Google Scholar]
- Ward DS, Bellville JW. Reduction of hypoxic ventilatory drive by dopamine. Anesth Analg. 1982;61:333–337. [PubMed] [Google Scholar]
- Ward S, Ryan S, Mc Nicholas WT, Heneghan C. Comparison of baroreflex sensitivity measures for assessing subjects with obstructive sleep apnea. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3572–3575. doi: 10.1109/IEMBS.2006.259621. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest. 1978;61:708–713. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.