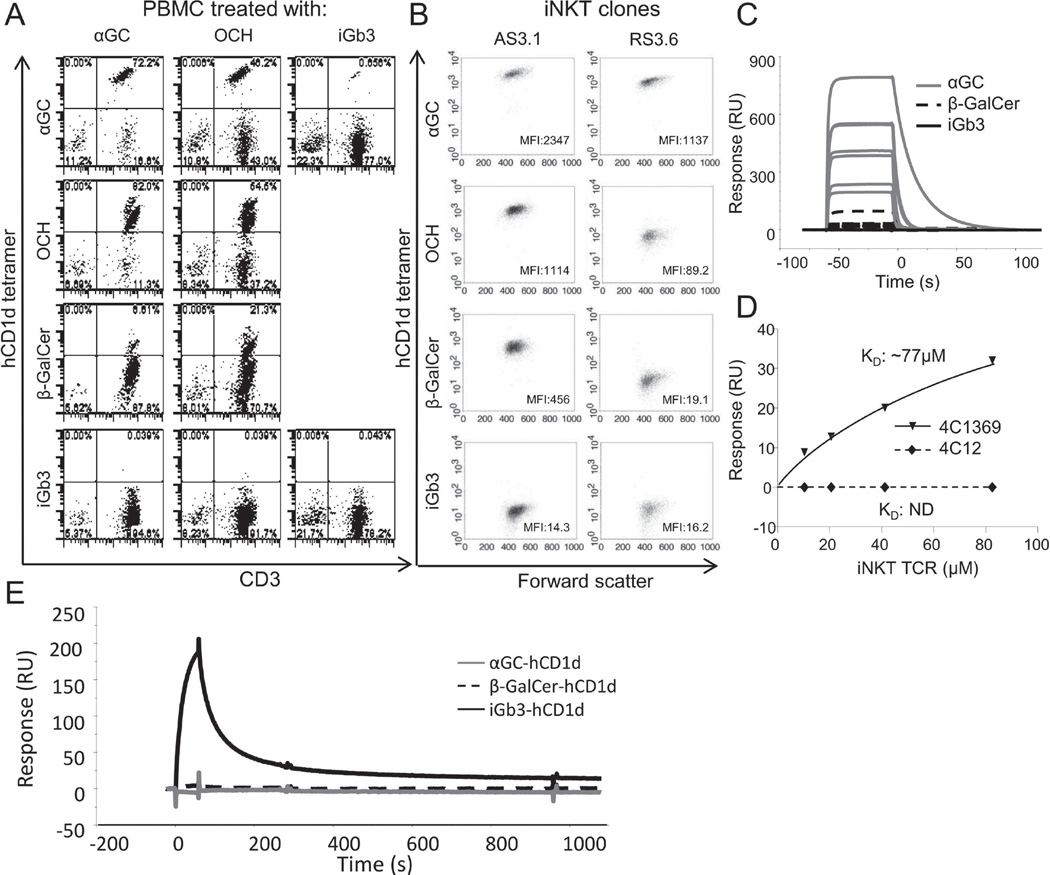
Figure 2.
Human iNKT TCRs do not bind to the hCD1d-iGb3 complex. (A) Binding of fluorescent-conjugated hCD1d tetramers generated by in vitro refolding using either αGC, OCH or β-GalCer (rows 1–3) to (A) human iNKT cells in different lipid antigen-treated PBMC lines (columns 1–3), and (B) to two human iNKT clones of different TCR affinity (AS3.1, high affinity TCR; RS3.6, low affinity TCR). (C) Binding of seven different recombinant human iNKT TCRs at 1 µM concentration to chip-immobilised recombinant hCD1d-αGC (solid grey line), hCD1d- β-GalCer (dotted black line) and hCD1d-iGb3 (solid black line). Figure shows overlaid sensograms from surface plasmon resonance. (D) Binding of a high affinity iNKT TCR (4C1369) and a low affinity iNKT TCR (4C12) to hCD1d-iGb3. Concentration response curves display binding of both TCRs to chip-bound hCD1d-iGb3. (E) Overlaid surface plasmon resonance sensograms showing binding of IB4 lectin to chip-immobilised recombinant hCD1d-iGb3 (black line), hCD1d-β-GalCer (dotted black line) or hCD1d-αGC complexes (grey line). All data shown are representative of two or more independent experiments performed.