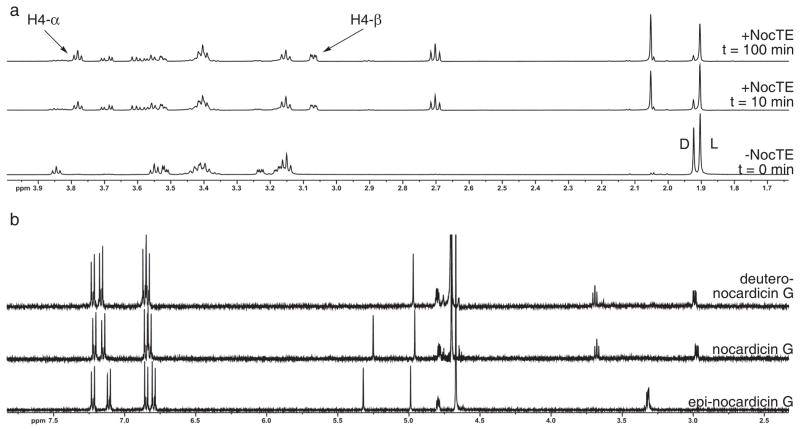
Figure 4. 1H-NMR spectrometric analysis of NocTE with epi-nocardicin G/nocardicin G-SNAC.
(a) 1H-NMR experiment demonstrating NocTE turnover of epi-nocardicin G/nocardicin G-SNAC. The bottom spectrum labeled “-NocTE” shows the substrates 11a/b before NocTE addition. “D” and “L” correspond to the N-acetyl peaks of SNAC of the nocardicin G-SNAC (11b) and epi-nocardicin G-SNAC (11a) thioesters, respectively. Arrows highlight the two C-4 diastereotopic hydrogen resonances (H-4α/β) of 1, whose appearance was monitored throughout the experiment. (b) 1H-NMR comparison of synthetic epi-nocardicin G and nocardicin G with isolated deutero-nocardicin G from NocTE conversion of substrate 11a/b in D2O.