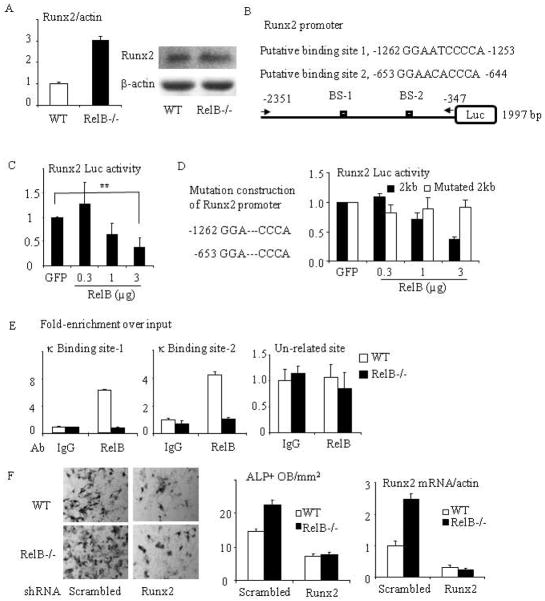
Fig. 5. RelB directly targets the Runx2 promoter and inhibits Runx2 expression.
(A) mRNA (left panel) and protein (right panel) expression of Runx2 were tested by Real-time PCR and Western blot from RelB−/− and WT BM stromal cells cultured with OB differentiation medium for 7d. (B) Construction scheme of mouse Runx2 promoter luciferase (Luc) reporter. The 2-kb Runx2 promoter constructs contain 2 putative κB binding sites. (C) The 2-kb Runx2 promoter Luc reporter was co-transfected with RelB plasmid into C2C12 cells and the relative Luc activity was tested. (D) Site-directed mutagenesis of both κB binding sites 1 and 2 in the 2 kb Runx2 promoter was performed by deleting “ATC” and “ACA”. A luciferase activity assay was performed using C2C12 cells that were co-transfected with a RelB plasmid and the mutated Runx2 reporter. (E) ChIP assays were carried out using an anti-RelB or control IgG antibody on sheared chromatin from WT and RelB−/− MPCs. Immunoprecipitated DNA was analyzed by qPCR using primers covering either NF-κB binding site-1 (left panel) or 2 (middle panel) in the Runx2 promoter region or a pair of un-related primers (right panel) designed in the region that is 3 kb apart from the κB binding sites. Results are expressed as fold-enrichment compared with IgG normalized to input. (F) bMPCs from WT and RelB−/− mice were transfected with a scrambled or Runx2 mouse shRNA sequence for 2 days followed by puromycin selection to kill the uninfected cells. The cells were treated with OB differentiation medium for 5d and stained for ALP activity to measure ALP+ cells (left and middle panel), and mRNA expression of Runx2 in these cells was tested by real-time PCR (right panel). All mice in in vitro experiments were 1.5 to 2 months-old. * p<0.05; ** p<0.01 vs. control.