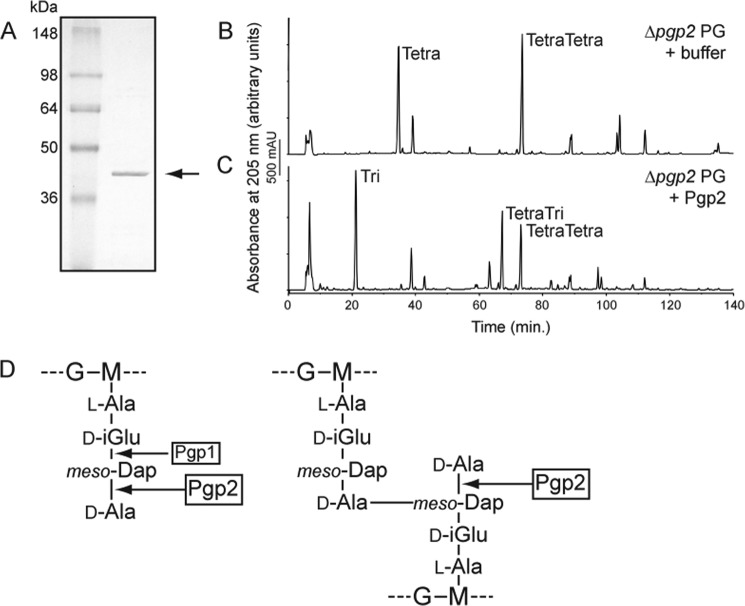
FIGURE 4.
Pgp2 has ld-carboxypeptidase activity on Δpgp2 PG, cleaving monomeric and cross-linked disaccharide tetrapeptides to tripeptides. A, SDS-PAGE analysis of affinity-purified Pgp2 with a predicted molecular mass of 37.0 kDa, indicated by an arrow. Shown are HPLC chromatograms of Δpgp2 PG (B) and Δpgp2 PG incubated with purified Pgp2, followed by cellosyl digestion and reduction with sodium borohydride (C). Peaks corresponding to monomeric disaccharide tripeptide (Tri) and disaccharide tetrapeptide (Tetra) and dimeric bis-disaccharide tetratripeptide (TetraTri) and bis-saccharide tetratetrapeptide (TetraTetra) are indicated. D, schematic diagram of the Pgp1 (determined in Ref. 11); Pgp2 carboxypeptidase cleavage sites are indicated with an arrow. Note that Pgp2 hydrolyzes tetrapeptides, and Pgp1 hydrolyzes tripeptides. G, N-acetylglucosamine; M, N-acetylmuramic acid; d-iGlu, d-isoglutamic acid.