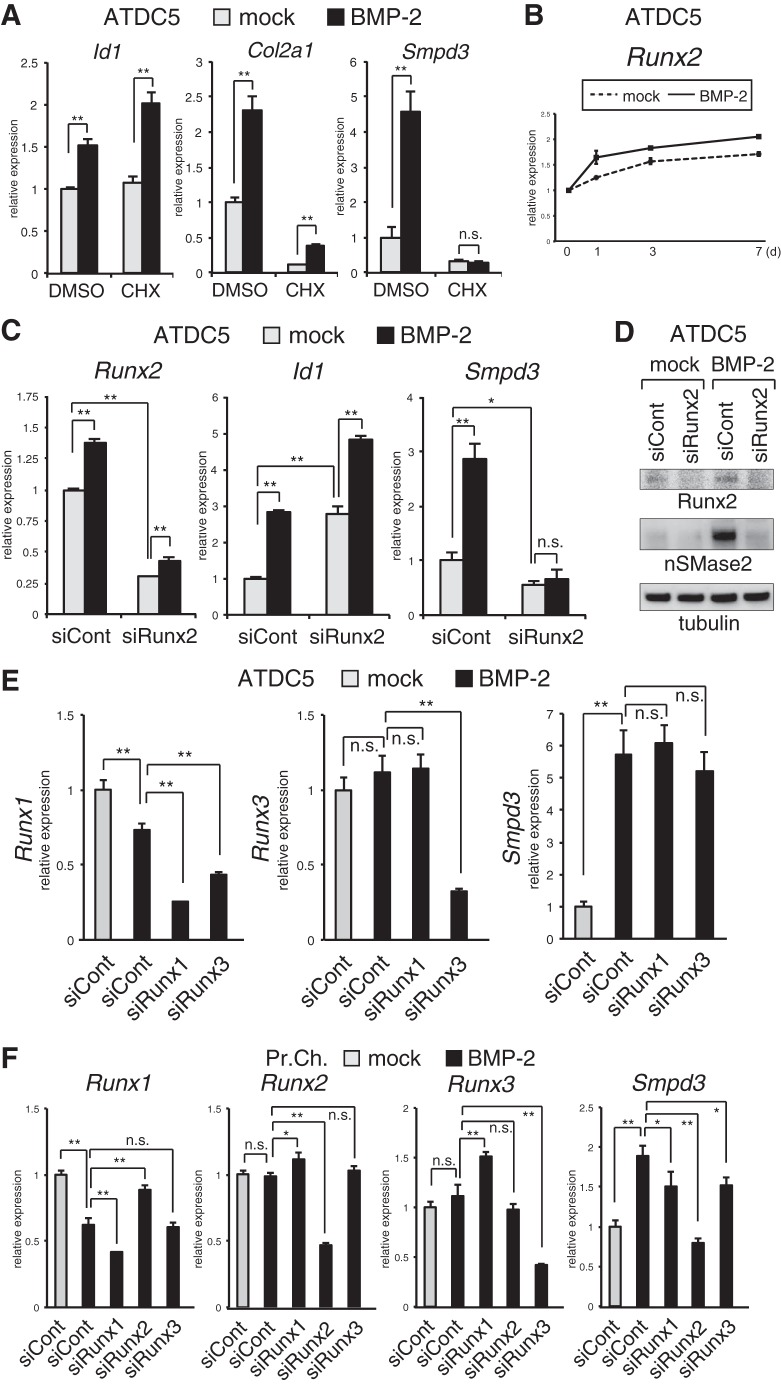
FIGURE 2.
BMP-2-induced increase of Smpd3 expression in chondrocytes is Runx2-dependent. A, CHX was applied to ATDC5 cells at a concentration of 10 mm for 2 h prior to BMP-2 (300 ng/ml) stimulation. Cells were harvested 24 h after BMP-2 induction to perform quantitative RT-PCR analysis for Id1, Col2a1, and Smpd3. B, ATDC5 cells were cultured with BMP-2 (300 ng/ml) for the indicated times. Expression of Runx2 was examined by quantitative RT-PCR. C, ATDC5 chondrocytes were transfected with control siRNA (siCont) or Runx2 siRNA (siRunx2) for 16 h and then treated with or without BMP-2 (300 ng/ml) for 48 h. Quantitative RT-PCR analysis was performed for Runx2, Id1, and Smpd3. D, ATDC5 cells were transfected with control siRNA (siCont) or Runx2 siRNA (siRunx2) for 16 h and stimulated with BMP-2 (300 ng/ml) for 48 h. Cells were subjected to immunoblot analysis for the indicated antibodies. Tubulin served as a loading control. E, ATDC5 cells were transfected with control siRNA (siCont), Runx1 siRNA (siRunx1), or Runx3 siRNA (siRunx3) for 16 h and then treated with or without BMP-2 (300 ng/ml) for 48 h. Quantitative RT-PCR analysis was performed for Runx1, Runx3, and Smpd3. F, mouse primary chondrocytes were transfected with control siRNA (siCont), Runx1 siRNA (siRunx1), Runx2 siRNA (siRunx2), or Runx3 siRNA (siRunx3) for 16 h and then treated with or without BMP-2 (300 ng/ml) for 48 h. Quantitative RT-PCR analysis was performed for Runx1, Runx2, Runx3, and Smpd3. *, p < 0.05; **, p < 0.01; n.s., not significant.