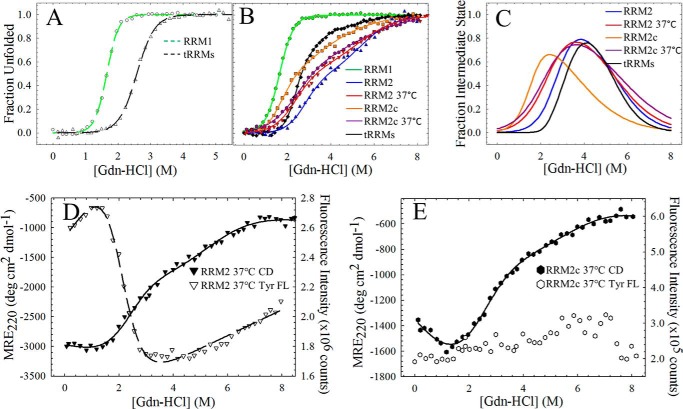
FIGURE 4.
Cleavage and physiological temperatures destabilize RRM2 and increase the population of the intermediate state. A, fraction apparent plot of the Trp FL of RRM1 (open circles, green dashed line) and tRRMs (open triangles, black dashed line) at 20 °C reveals a shift toward higher denaturant for RRM1 unfolding upon tethering to RRM2. B, fraction apparent plots of all TDP-43 RRM constructs by CD. RRM1 displays a two-state equilibrium profile (green), whereas RRM2 (20 °C, blue; 37 °C, red) and the cleavage fragment, RRM2c (20 °C, orange; 37 °C, purple) display a three-state profile with the population of an intermediate state. tRRMs (black) display a complex unfolding profile with the population of two stable intermediates. C, RRM2 intermediate population as a function of denaturant using Equation 3 for a three-state profile (RRM2 and RRM2c) or Equation 4 for a four-state profile (tRRMs). Increasing temperature (red) or cleavage (orange and purple) within the RRM2 domain (blue) results in an increased intermediate population under native conditions (0 m GdnHCl) with a shift to lower denaturant for the maximum intermediate population. Tethering RRM2 to RRM1 (black) reduces the RRM2 intermediate state under native conditions and shifts the maximum population to higher denaturant. D, physiological temperatures destabilize the N ⇆ I transition by CD (filled inverted triangles, solid line) and Tyr FL transition (open inverted triangles, dashed line) with a shift in the Cm to lower denaturant compared with RRM2. E, physiological temperature results in a similar unfolding profile for RRM2c to RRM2 at 37 °C (compare filled inverted triangles with filled hexagons). Tyr FL revealed little change between the folded and unfolded states (open hexagons), suggesting that Tyr214 resembles the unfolded state at this temperature. Each experiment was consistently reproduced three times.