FIGURE 2.
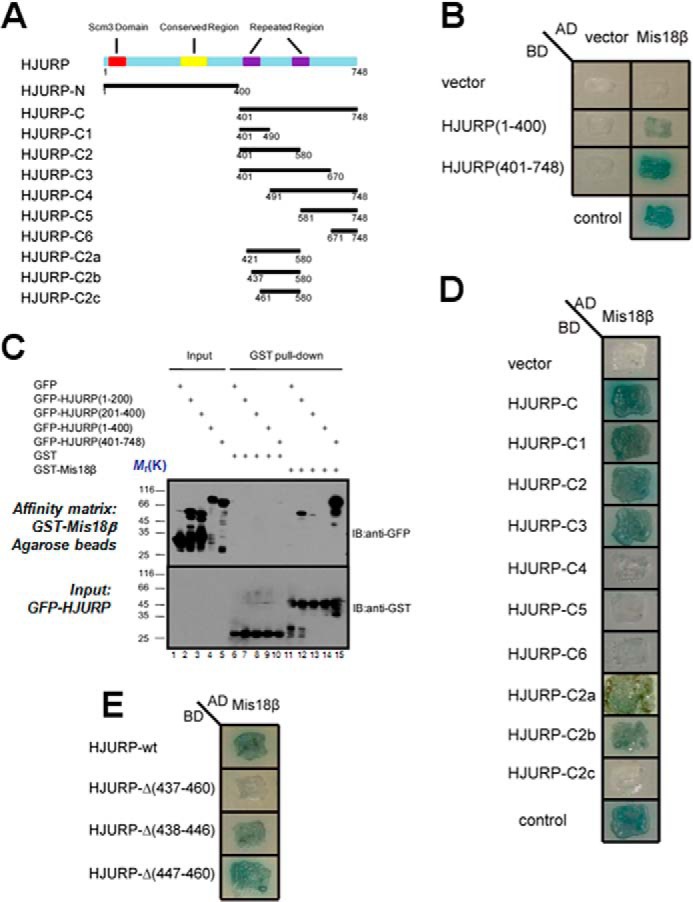
Identification of structural determinant of HJURP essential for binding to Mis18β. A, schematic representations of the domain organization of HJURP and constructs of truncations. B, AD-Mis18β, together with two BD constructs as indicated, were co-transformed into yeast cells and cultured on a selective plate containing X-α-Gal but lacking uracil, tryptophan, leucine, and histidine. Control is the cells co-transformed with BD-p53 and AD-T as a positive control. C, purified GST-Mis18β was immobilized on glutathione-agarose and incubated with extracts of 293T cells expressing GFP-tagged HJURP fragments. After extensive wash, proteins associated with glutathione-agarose were eluted and separated by SDS-PAGE followed by transfer to nitrocellulose membrane for Western blotting analyses (IB) with anti-GFP and anti-GST antibody, respectively. D and E, AD-Mis18β, together with different BD constructs of different HJURP truncations, were co-transformed into yeast cells and cultured on selective plate containing X-α-Gal but lacking uracil, tryptophan, leucine, and histidine. Control is the cells co-transformed with BD-p53 and AD-T as a positive control. Note that deletion of 24 amino acids (aa 437–460) from HJURP abolished the interaction between HJURP and Mis18β.
