FIGURE 7.
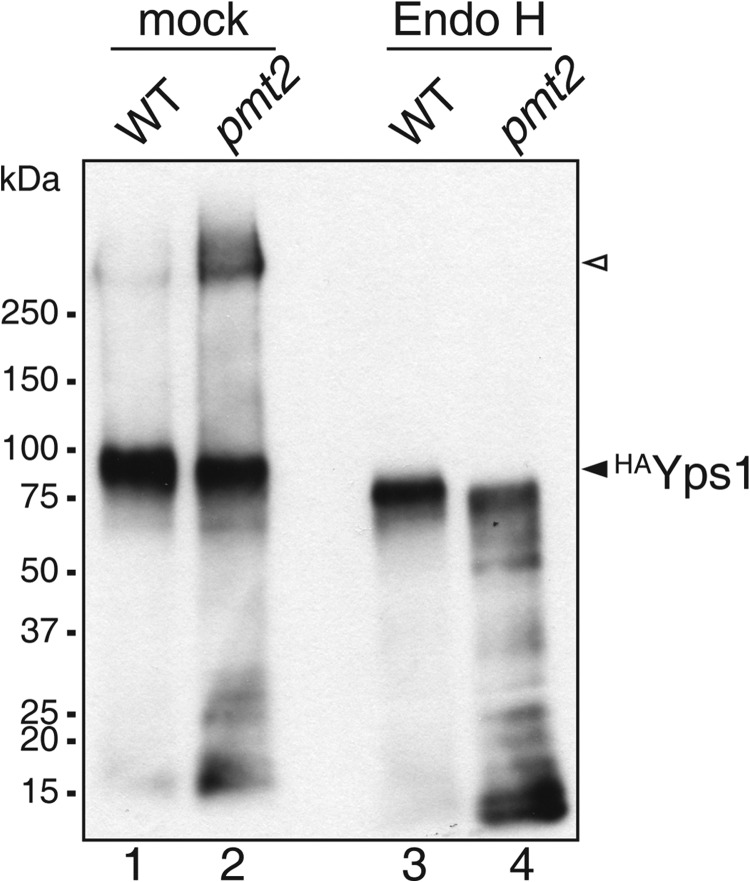
Glycosylation of Yps1 in WT and mutant pmt2. HAYps1 is aberrantly N-glycosylated in pmt2 mutant cells. Cell walls were isolated from WT and pmt2 mutant cells transformed with pJH33 (HAYps1). Proteins were extracted with SDS, treated with Endo H, resolved on SDS-polyacrylamide gels, and analyzed by Western blotting. Extracts from 1.5 × 107 cells were analyzed per lane. A prominent band was detected in the WT sample (∼90 kDa, lane 1), which shifted to lower molecular mass (∼75 kDa, lane 3) upon Endo H treatment indicating moderate N-glycosylation. In the pmt2 sample the ∼90-kDa form was less abundant and a high molecular mass form (∼270–350 kDa, open triangle) was detected (lane 2). Endo H treatment demonstrated that the high molecular weight form of HAYps1 in pmt2 cells was due to increased N-glycosylation (lane 4).