FIGURE 4.
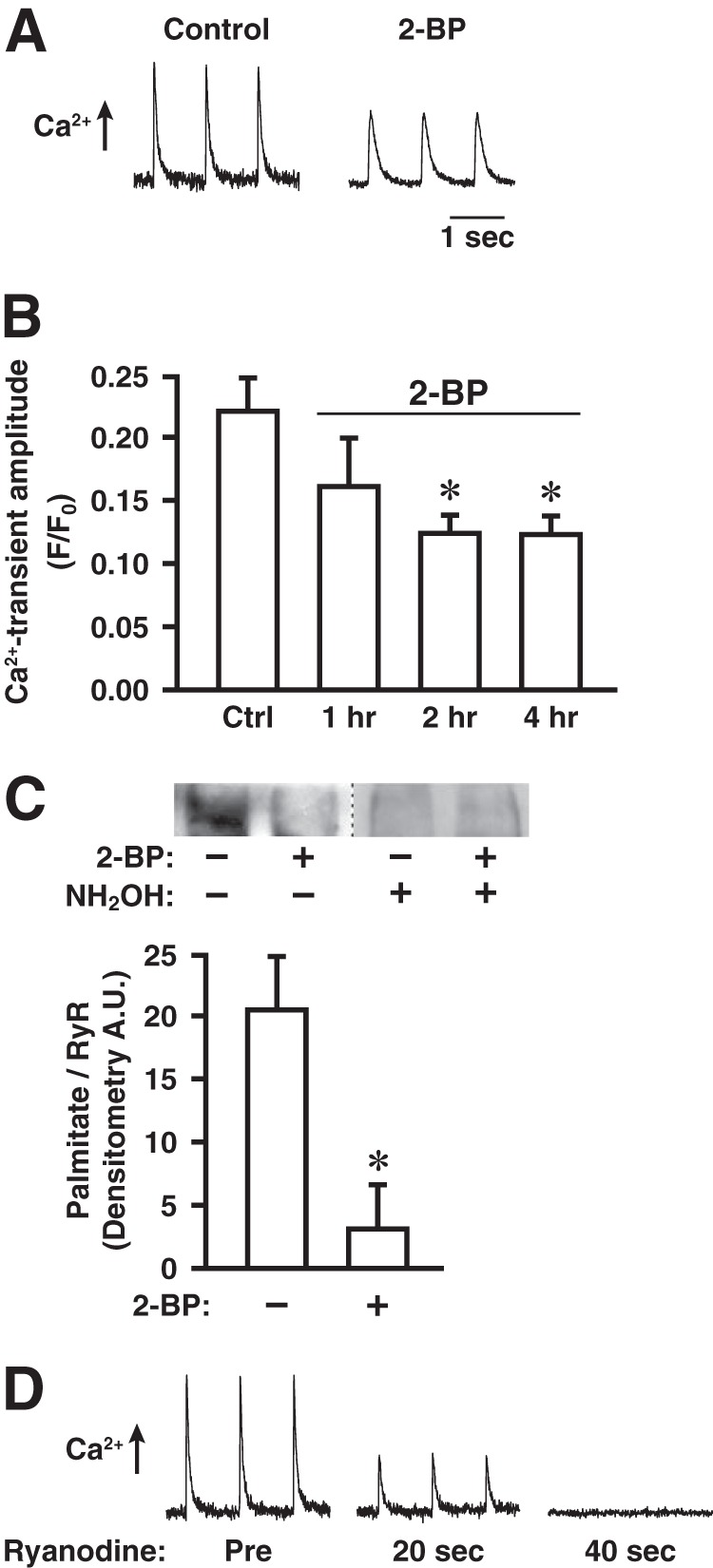
Treatment with 2-BP diminishes electrically induced Ca2+ release through RyR1 and turnover of palmitate on RyR1 in cultured myofibers. A, representative records of electrically induced (pacing at 1 Hz) intracellular Ca2+ release in a control myofiber and a myofiber treated with 2-BP (1 μm, 2 h), as assessed by Fluo 3-AM fluorescence. B, effects on electrically induced Ca2+ release of treatment with 2-BP (1 μm) for the indicated times (n = 4 separate experiments, 8–10 control and 8–10 2-BP-treated myofibers imaged per experiment; *, p < 0.05 with respect to control). Error bars indicate S.E. C, top, metabolic labeling with the ω-alkynyl-palmitate analog 17-octadecynoic acid (2 h) followed by detection of incorporated analog by bio-orthogonal click chemistry with biotin-azide demonstrating dynamic palmitoylation of at least some sites within RyR1 under basal conditions, which is greatly diminished by co-incubation with 2-BP (1 μm). Hydroxylamine (NH2OH)-sensitivity verifies that labeling represents thioester-linked palmitoylation. Bottom, quantification of the degree of RyR1 palmitoylation (normalized for RyR1 content of samples) demonstrating a decrease of approximately 85% in analog incorporation into RyR1 by co-incubation with 2-BP (*, p = 0.01; n = 4 separate experiments). Error bars indicate S.E. D, electrically induced Ca2+ release rapidly suppressed by inhibiting RyR1 with ryanodine (1 μm). Tracings shown are representative of three separate experiments.
