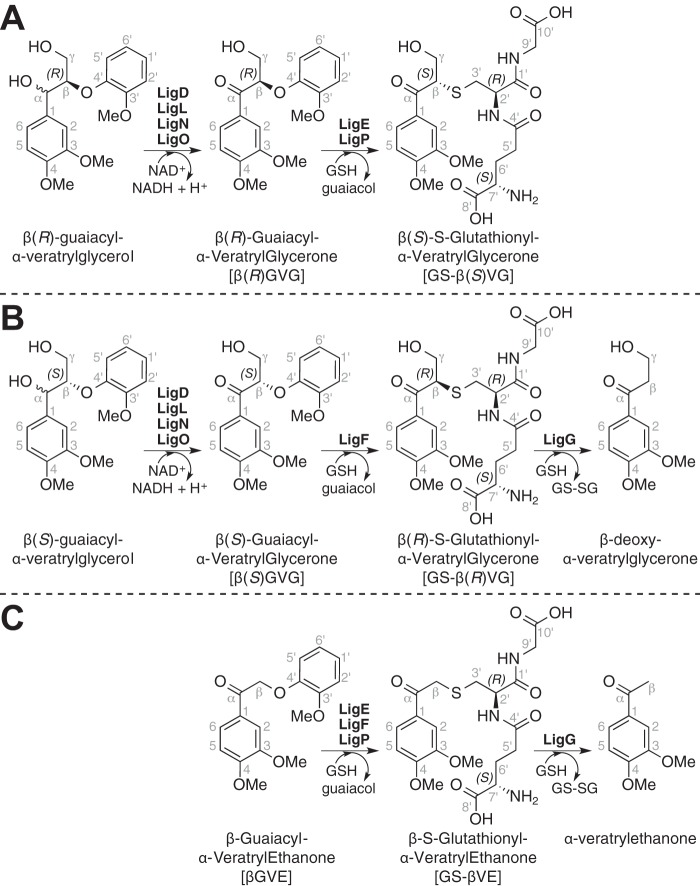
FIGURE 1.
The proposed β-etherase pathway in Sphingobium sp. strain SYK-6. Panels (A) and (B) show pathways for metabolism of β(R)- and β(S)-configured β-aryl ether model compounds, with Lig dehydrogenases (LigD, LigL, LigN, and LigO) catalyzing oxidation of the benzylic alcohol in model substrates β(R)- and β(S)-guaiacyl-α-veratrylglycerol, producing β(R)GVG and β(S)GVG. In the presence of GSH, βGVG enantiomers are cleaved to guaiacol and one of two GS-conjugated β-epimers, GS-β(S)VG or GS-β(R)VG, by either LigE, LigP, or LigF. Subsequent GSH-dependent cleavage of GS-β(R)VG by LigG generates β-deoxy-α-veratrylglycerone and GS-SG. C shows the reaction products of Lig enzymes when the achiral model compound βGVE is used as a substrate. In this case, the product of β-etherase activity is GS-βVE, and the product of GS-βVE thioether cleavage by LigG is α-veratrylethanone.