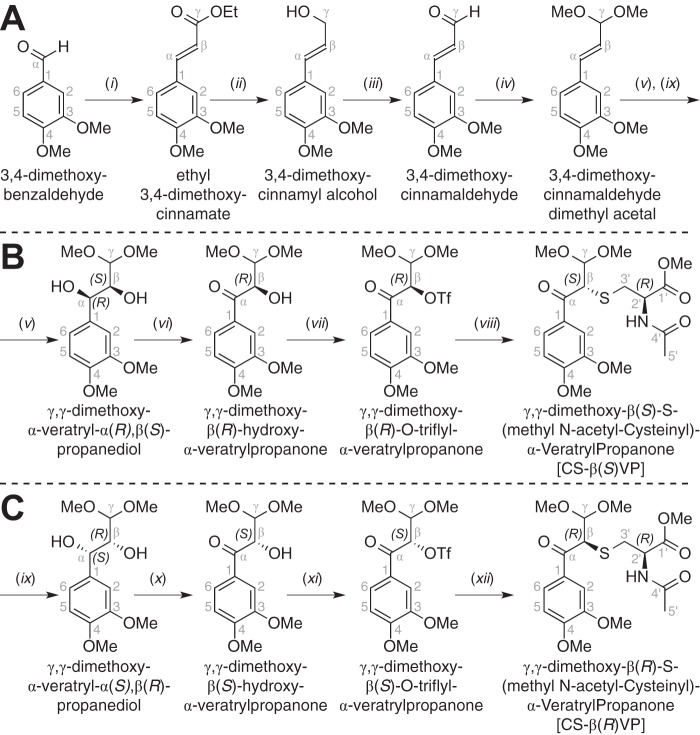
FIGURE 4.
Scheme for the synthesis of 3,4-dimethoxycinnamaldehyde dimethyl acetal (A), CS-β(S)VP (B), and CS-β(R)VP (C). Reagents and conditions were as follows: triethyl phosphonoacetate, NaH, THF, 2 h, 91% (i); DIBAL-H, THF, 2 h, 89% (ii); DDQ, 1,4-dioxane, 30 min, flash chromatography, 72% (iii); p-toluenesulfonic acid, trimethyl orthoformate, MeOH, 2 h, 96% (iv); AD-mix β, methanesulfonamide, 1:1 t-butanol/water, 4 °C, 18 h, 72% (v); DDQ, 1,4-dioxane, 30 min, flash chromatography, 73% (vi); trifluoromethanesulfonic anhydride, 2,6-lutidine, CH2Cl2, 2 h, flash chromatography, 75% (vii); methyl N-acetyl-(R)-cysteinate, K2CO3, dimethyl formamide, 2 h, 53% (viii); AD-mix α, methanesulfonamide, 1:1 t-butanol/water, 4 °C, 18 h, 83% (ix); DDQ, 1,4-dioxane, 30 min, flash chromatography, 81% (x); trifluoromethanesulfonic anhydride, 2,6-lutidine, CH2Cl2, 2 h, flash chromatography, 59% (xi); methyl N-acetyl-(R)-cysteinate, K2CO3, dimethyl formamide, 2 h, 73%. See supplemental material for details.