FIG. 2.
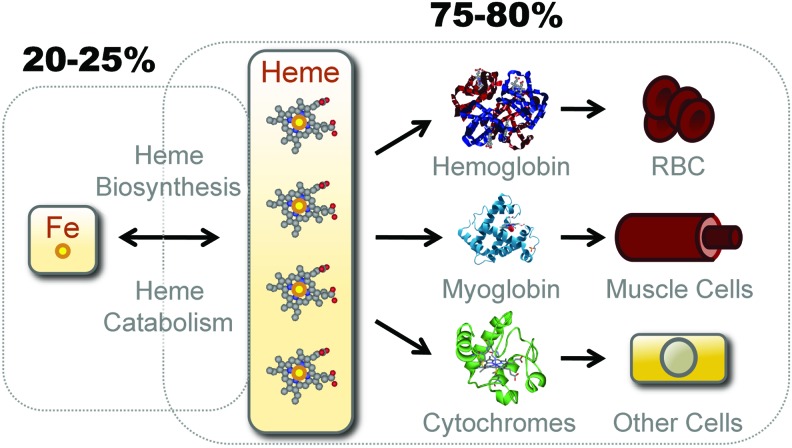
Bioavailable Fe. Only an estimated 20%–25% of the bioavailable Fe in mammals exists in the form of nonheme (labile) Fe, either bound to TF, stored by ferritin, as part of a Fe-Sulfur cluster or transiently bound to Fe chaperones and transporters. The remaining 75%–80% of the bioavailable Fe is contained inside the protoporphyn IX ring of heme as prosthetic groups of hemoproteins. The major hemoproteins compartments in mammals are hemoglobin (Hb) in red blood cells (RBCs), myoglobin in muscle cells, and cytochromes in virtual all cell types. Hb contains an estimated 70% of the total pool of heme, myoglobin 5%–10%, and cytochromes 25%–20%, respectively. Fe can transit from the “nonheme” to the “heme” compartment through de novo heme synthesis, a process driven by a sequence of eight enzymatic steps, the last being catalyzed by ferrochelatase, which inserts Fe inside the proptoporphyrin IX ring giving rise to heme. Fe can also transit from the heme to the nonheme pool via the catabolism of heme by HO enzymes or via heme oxidation, which releases Fe from the proptoporphyrin IX ring of heme. HO, heme oxygenases. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars