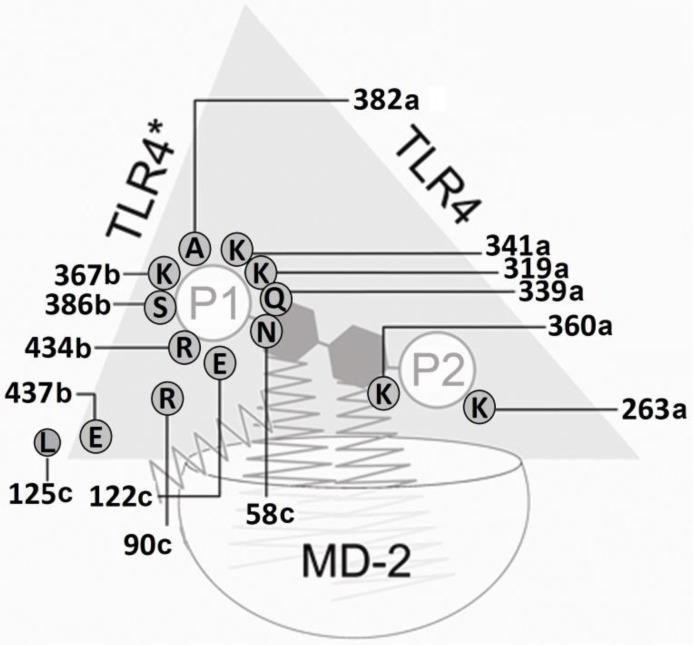
Figure 7.
Schematic view of the computed side chain interactions of the murine TLR4 ectodomain/MD-2 complex with Lipid IVA pointing out the agonistic action of Lipid IVA in the mouse system. The repulsive, anionic sidechain interaction of a glutamate residue specifically present in murine MD-2 (Glu122c) favors the upward shift of the phosphate residue P1 (1-PO4) at GlcN-I into the bridging position formed by a cluster of residues of TLR4, MD-2 and TLR4*. In line with the current consensus model of agonist-induced TLR4/MD-2 activation dimerization into the active m-shaped receptor complex takes place in consequence.