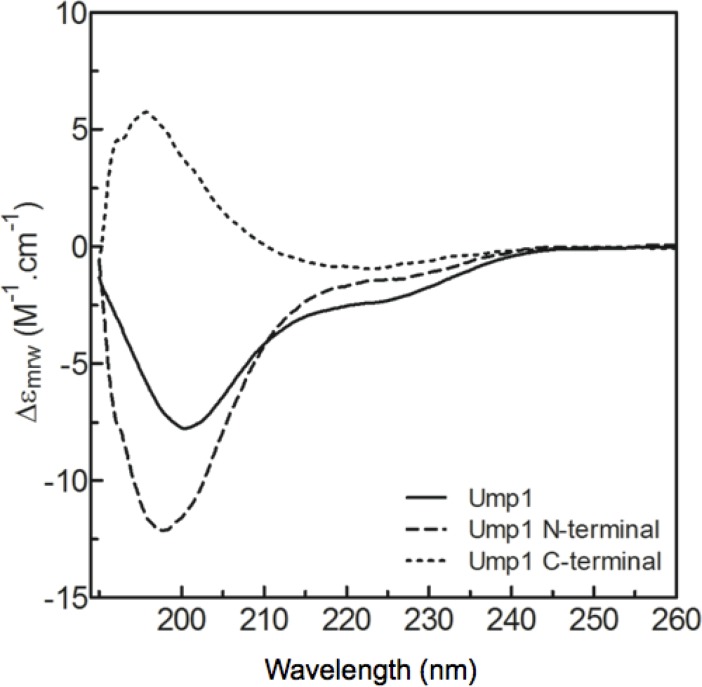
Figure 5.
The N-terminal region of Ump1 is highly disordered. Far-UV CD spectra of Ump1-C115S and its isolated N-terminal fragment. The difference spectrum for the C-terminal peptide was obtained by subtracting the N-terminal Ump1 spectrum from that of full-length Ump1-C115S. Upon deconvolution the secondary structure of Ump1 N-terminal is 1% α-helix, 25% β-strand, 22% turns and 50% coil. The C-terminal peptide secondary structure corresponds to 18% α-helix, 37% β-strand, 19% turns and 27% coil.