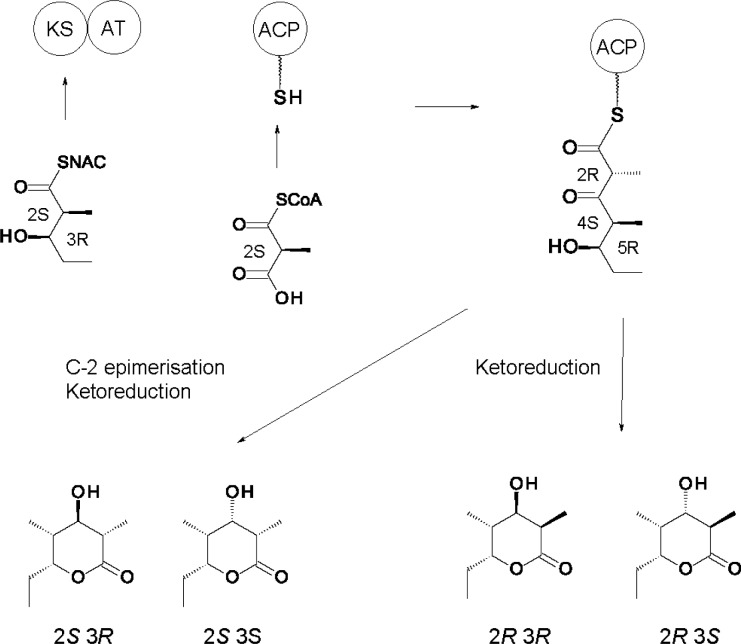
Figure 2.
Synthesis of triketide lactones using discrete ACPs and KS-AT didomains. (2S, 3R)-2-methyl-3-hydroxypentanoyl-NAC is used to acylate the KS domain. The AT uses (2S)-methylmalonyl CoA to load the ACP. Condensation proceeds with inversion to give a (2R)-2-methyl-3-ketoacyl-ACP. Triketide lactone products are identified by GC-MS. Reduction of the ketone with borohydride prior to chain release gives a racemic mixture of (3R)- and (3S)- alcohols but fixes the (2R) methyl stereochemistry. Stereospecific ketoreduction can be achieved by adding a KR and NADPH.